Introduction
Gyrate atrophy (GA) of the choroid and retina is a rare genetic disease of autosomal recessive inheritance. It primarily affects the ocular tissues and occurs due to deficiency of the enzyme ornithine aminotransferase (OAT) that leads to a 10 to 20 times increase in the plasma level of the amino acid ornithine, compared to the normal plasma levels, which is thought to result in the ocular manifestations associated with the condition.[1][2] This occurs due to mutations in the OAT gene located on chromosome 10, which results in a decrease or absence of the activity of the enzyme OAT.[3][4] The condition is characterized by the development of chorioretinal atrophic patches that start in the mid-peripheral retina in the first decade and spread centrally to the macular area, myopia, changes in the macula including cystic changes that appear in the first and second decades, and posterior subcapsular cataracts.[5][6] Patients usually present with night blindness followed by visual field constriction and eventually diminution of central vision and blindness.[5][7] The condition is diagnosed by the presence of the characteristic clinical picture, the presence of hyperornithinemia in plasma, and the detection of mutations in the OAT gene.[8] Treatment mainly involves dietary modifications and management of complications.[9][10][11][6]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
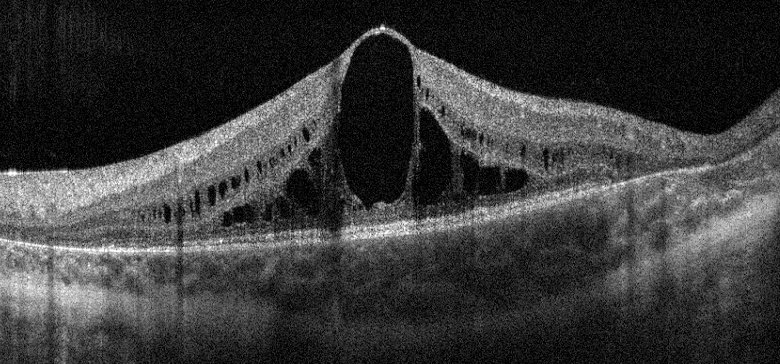
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Gyrate atrophy of the choroid and retina is a rare genetic condition of autosomal recessive inheritance that results from mutations affecting the OAT gene on chromosome 10q26, leading to deficiency of the pyridoxal-dependant mitochondrial enzyme OAT which normally metabolizes the amino acid ornithine into pyrroline-5-carboxylic acid.[12][13] Currently, there are more than 60 reported mutations in the OAT gene that lead to GA.[4][14] Defective ornithine metabolism leads to the accumulation of excessive ornithine in the plasma, urine, cerebrospinal fluid, and aqueous humor which is postulated to result in the manifestations of the condition possibly due to the toxic effects of hyperornithinemia, especially on the retinal pigment epithelial cells.[15] The condition can also be associated with excessive excretion of lysine and cystine in the urine with a decrease in the plasma levels of lysine, glutamine, glutamic acid, ammonia, and creatine.[16][17] Although the OAT enzyme is expressed in most tissues, the main pathological manifestations of the condition are those involving the eye.[16][18]
Epidemiology
Gyrate atrophy is a rare condition that, for unknown reasons, is reported to be particularly prevalent in Finland but has been reported in many other countries of the world including the USA, Japan, Germany, UK, India, China, Australia, France, Tunisia, Egypt, Korea, Brazil, Nepal, and Turkey.[5][19][7][20][21][22][23][24][25][26][6][27][28][29][30] In Finland, the prevalence is estimated to be around 1 in 50,000.[31] Myopia, night blindness, and visual field affection associated with the condition usually start in the first decade of life followed by a diminution of vision due to macular affection and cataract formation that usually follows in the second decade, however, the onset of central vision loss is highly variable and can occur at any age according to a Finnish study.[5] Some studies have shown that females may retain larger visual fields when compared to males, with preservation of night vision; however, other studies have shown that the visual field is equally affected in both males and females.[5][32][7] In a study comparing the natural history of Japanese patients to Finnish patients, there was a worse visual function in Japanese patients, which suggests that the natural history of the condition could be variable among patients from different populations.[7] Patients, however, usually have worse than 20/200 vision between 40 and 55 years of age.[5]
Pathophysiology
Deficiency of the pyridoxal-dependant mitochondrial enzyme OAT, which normally metabolizes the amino acid ornithine into pyrroline-5-carboxylic acid, leads to accumulation of ornithine in the plasma, urine, cerebrospinal fluid, and aqueous humor of patients with GA which is thought to result in the manifestations of the condition possibly due to the toxic effects of hyperornithinemia, especially on the retinal pigment epithelial cells, leading to progressive chorioretinal atrophy.[12][13][15] Indeed, in a mouse model of GA, there was histopathologic evidence that the RPE is the initial site of damage in GA.[33] OAT is expressed at high levels in the RPE and a lower level in the photoreceptors, and the RPE could be especially sensitive to ornithine accumulation in the case of OAT deficiency.[33][34][35] RPE damage and atrophy may then lead to choriocapillaris atrophy leading to the characteristic chorioretinal degenerative patches seen in GA.[36] Damage to photoreceptors in patients with GA could be the result of a combination of mechanisms, including direct toxicity from hyperornithinemia, damage from toxic residues produced by degenerating RPE cells, the inability of RPE cells to provide normal nutrition to the photoreceptors, reduced nutrition due to atrophy of the choriocapillaris, and breakdown of the blood-retinal barrier leading to an impaired photoceptor microenvironment that affects their normal function.[33] This leads to a progressive deterioration of the visual field and, eventually, central visual acuity.
Other biochemical findings associated with GA include excessive excretion of lysine and cystine in the urine with a decrease in the plasma levels of lysine, ammonia, glutamine, and creatine.[16][37] Several manifestations of GA, particularly central nervous system manifestations such as mental retardation and epilepsy, together with the associated muscle findings, may occur due to the secondary phosphocreatine deficiency that results from the inhibition of creatine synthesis due to the hyperornithinemia.[38][39] This energy deficiency may also affect the function of the RPE, leading to chorioretinal atrophy.[38]
Histopathology
Histopathologic studies of GA are rare. In a postmortem study of a patient with pyridoxine-responsive GA, the retina showed focal areas of atrophy of the photoreceptors with hyperplasia of the adjacent RPE. An abrupt transition from the almost-normal retina to the zone of the almost totally atrophic retina, RPE, and choroid was present in the retinal mid-periphery. Examination by electron microscopy revealed abnormalities in the mitochondria of the corneal endothelium and the non-pigmented epithelium of the ciliary body. There were also similar, albeit less severe, mitochondrial abnormalities affecting the photoreceptors.[40]
In an adult domestic cat with a condition analogous to GA characterized by OAT deficiency and plasma hyperornithinemia, a postmortem study revealed atrophy of the RPE and photoreceptors throughout the fundus with an abnormal and discontinuous choriocapillaris layer.[41]
In a study of OAT-deficient GA mouse models developed by gene targeting, there was marked swelling of the RPE cells with irregular shape and engorgement in mice on a standard diet. There was also an absence of the outer segments of photoreceptors, while the inner segments were disorganized and shortened. The outer nuclear layer was also markedly reduced in thickness. These changes were not apparent in mice on an arginine-restricted diet. The findings were also confirmed by electron microscopy.[15]
Muscle biopsy of patients with GA typically shows gross fatty changes with type 2 muscle fiber atrophy and tubular aggregates.[42]
History and Physical
Patients with gyrate atrophy of the choroid and retina usually present with night blindness and progressive visual field constriction in their first decade of life that occurs due to progressive peripheral chorioretinal degeneration.[5] A family history of the condition may sometimes be present and lead to the early detection of the condition through screening in early childhood. Gradually progressive diminution of central visual acuity usually follows these manifestations, occurring later in the first or second decade due to the development of macular changes and posterior subcapsular cataracts.[5][6] Macular changes associated with GA include intraretinal cystic spaces, cystoid macular edema, foveoschisis, epiretinal membrane, and atrophy.[6][43][26] Myopia is also a commonly associated feature with the condition. Complications such as a macular hole and subfoveal choroidal neovascularization can also occur but lead to a more severe and acute diminution of central visual acuity.[44][45] Most patients will usually become legally blind (vision less than 20/200) between 40 and 55 years of age due to the late macular involvement by the chorioretinal atrophy.[5]
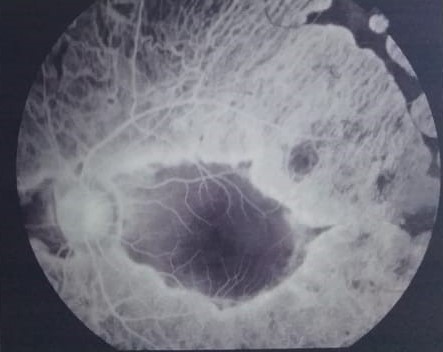
On ophthalmoscopy, the peripheral chorioretinal degeneration appears as discrete scalloped areas of chorioretinal atrophy that progressively coalesce together and have pigmented edges with visible large choroidal vessels underneath.[1] These atrophic patches usually start in the retinal mid-periphery but later spread to the macular area centrally and to the ora serrata peripherally to involve the whole retina with resultant visual field deterioration.[1][5] They can also be associated with peripapillary atrophy. Retinal crystals may also be present.[1] The normal foveal reflex may not be apparent due to the associated macular cystic changes. Cataract associated with GA is usually of the posterior subcapsular type and is best appreciated by the slit-lamp biomicroscopy or direct ophthalmoscopy using the red reflex. Choroidal neovascularization is suspected by the presence of a subretinal greyish-yellow lesion with surrounding subretinal fluid, hemorrhage, and exudates.
Although ophthalmic features are the main manifestations of GA, several central nervous system manifestations may also be associated with the condition, including aggressive behavior, mental retardation, and epilepsy.[38] Peripheral nervous system abnormalities have also been reported, and patients may occasionally have muscular weakness.[46][39][42]
Evaluation
The diagnosis of gyrate atrophy of the choroid and retina depends on the presence of the characteristic clinical features of the condition and the presence of elevated plasma ornithine level, which is essential for the diagnosis of the condition and is usually 10 to 20 times higher than the normal plasma ornithine level.[1][2][8] Other laboratory findings include excessive excretion of lysine and cystine in the urine with a decrease in the plasma levels of lysine, glutamine, and creatine.[16] Deficiency of the OAT enzyme activity can also be demonstrated in cultured skin fibroblasts of patients, which may also be partially deficient in carriers.[13][47] There are currently more than 60 known mutations in the OAT gene that lead to GA, and the detection of these mutations can help confirm the diagnosis.[4][14] Because the condition is inherited in an autosomal recessive manner, it is important to screen the relatives of patients, especially their siblings, since early treatment may result in better long-term visual outcomes. Patients with GA have been found to have abnormally slow background electroencephalogram activity with focal and epileptogenic lesions, and some patients may also have mental retardation, aggressive behavior, or epilepsy.[38][48] Some patients also show degenerative lesions and atrophic changes in brain magnetic resonance imaging with severe creatine deficiency detected on brain magnetic resonance spectroscopy.[38][48] Electromyography may also be abnormal with a myopathic pattern, and muscle biopsy may show atrophy, which may also be related to creatine deficiency.[39]
Regarding the ophthalmic evaluation of the condition, fluorescein angiography of patients with GA shows prominent retinal pigment epithelium (RPE) transmission window defects which start in the peripheral retina and correspond to the areas of RPE and choriocapillaris atrophy that characterize the disease with a hyperfluorescent lining along the borders of these defects. Large choroidal vessels are clearly seen through these defects. These defects gradually enlarge in size on follow-up and progress to coalesce together.[5] Some patients also have cystoid macular edema associated with macular leakage. Others, such as patients with foveoschisis, do not have macular leakage.[22] Later, the macula can also be involved with chorioretinal atrophy.[1][49] Fluorescein angiography can also demonstrate leakage in cases of GA complicated by choroidal neovascularization.[50][51]
GA can also be evaluated and followed up using ultra-widefield imaging, which allows superior imaging of the retinal periphery simultaneously with the posterior pole.[22][52] Electroretinography (ERG) reveals diminished a and b wave responses of both rods and cones, which become undetectable in advanced disease; however, a nearly normal ERG in association with GA has also been reported.[21][53] There are also abnormalities detected in the electrooculography and dark adaptometry of patients.[54]
Visual field testing is useful in follow-up using both static and kinetic perimetry and shows progressive visual field constriction and loss of sensitivity with age, which can be slowed with appropriate treatment.[5][7][55] The visual field is also important for consideration for driving and to determine the visual disability status. Microperimetry, which applies targeted stimuli to discrete areas of the retina, may also be useful in testing macular sensitivity and function, which depends on the severity of macular affection.[56]
Fundus autofluorescence, which is an indicator of RPE structure, may help demonstrate and follow up areas of chorioretinal atrophy as characteristic hypoautofluorescent areas.[56]
Optical coherence tomography is useful in the evaluation of the macular area and can show variable features including intraretinal cystic spaces.[6] cystoid macular edema,[43] foveoschisis,[26] macular hole,[44] choroidal neovascularization,[45] epiretinal membranes,[56] foveal thinning,[26] outer retinal tubulations,[56] and choroidal thinning.[56] Optical coherence tomography angiography findings in patients with GA have been recently described and include superficial and deep retinal ischemia,[6] perifoveal deep retinal microvascular alterations,[26] enlargement of the foveal avascular zone at the level of the superficial and deep capillary plexuses that correlates with foveal cyst size,[57] and decreased macular vascular density.[58]
Treatment / Management
Treatment of gyrate atrophy consists mainly of dietary modifications to help lower the elevated systemic ornithine levels. The restriction of arginine in the diet, the precursor amino acid for ornithine, has been found to effectively lower plasma ornithine levels and to retard the progression of chorioretinal degeneration in both human and GA mouse models.[9][59][54][60][61] Some patients, however, continue to develop progressive chorioretinal degeneration and electroretinographic changes despite lowered plasma ornithine levels following arginine restriction, which could be due to the genetic heterogeneity associated with the condition.[62] Food rich in arginine that should be avoided or reduced in the diet includes nuts, seeds, cereal, dairy products, seafood, meat, chicken, watermelon, and chocolate.[37][63](B3)
Some patients may also benefit from vitamin B6 supplementation in lowering their plasma ornithine levels, which acts by increasing the activity of the pyridoxine-dependant OAT enzyme, while other patients do not.[47][64] These are known as pyridoxine responders and non-responders, respectively, which has been proven by both in vivo and in vitro methods.[60] This is possibly due to the different mutations that affect the OAT gene.[54][64][60] The dose of vitamin B6 supplementation used for patients with GA in studies is variable and has ranged from around 120 to 600 mg/day.[65][66](B3)
Creatine supplementation may also have a role in retarding the chorioretinal degeneration and in improving neurological and muscular manifestations.[54][67][68] Other dietary modifications that could be of benefit include proline and lysine supplementation.[7][54]
Examination of the fundus of the family members is very crucial to detect the disease in an early stage and to start intervention early so that the methods to reduce progression can be tried.
Refraction and low vision aid form an important part of management and may improve the quality of life of the patient.
Treatment of cystoid macular edema and intraretinal cystic spaces associated with GA includes restriction of arginine in diet, vitamin B6 supplementation,[69] topical and oral carbonic anhydrase inhibitors, topical non-steroidal anti-inflammatory drugs,[70][71] intravitreal or subtenon steroid injections which, however, carry the risk of cataract progression and intraocular pressure elevation, and intravitreal antivascular endothelial growth factor injections.[6][72][30][73][28][74] In cases of foveoschisis with no apparent macular leakage on fluorescein angiography, carbonic anhydrase inhibitors could be used.(B3)
Treatment of ocular complications of GA includes cataract surgery for visually significant cataract which may also be associated with zonular weakness,[5][75] pars plana vitrectomy for macular holes,[44] rhegmatogenous retinal detachments,[76] and vitreous hemorrhage,[77] and intravitreal antivascular endothelial growth factor injections for choroidal neovascularization.[45][50](B3)
Differential Diagnosis
The differential diagnosis of gyrate atrophy includes the following conditions:
- Choroideremia [78]
- Retinitis pigmentosa [79]
- Congenital stationary night blindness [80]
- X-linked retinoschisis [81]
- Bietti crystalline dystrophy [82]
- Pigmented paravenous retinochoroidal atrophy [83]
- Bifocal chorioretinal atrophy [84]
- Pathological myopia [85]
- Choroidal sclerosis [86]
- Gyrate atrophy-like phenotypes with normal plasma ornithine [87][88]
- Extensive paving stone (cobblestone) degeneration, which however is peripheral
These conditions can be differentiated from GA on the basis of the patient's history, inheritance pattern, clinical picture, laboratory findings, genetic analysis, electrophysiology, and multimodal imaging analysis.
Toxicity and Adverse Effect Management
Patients with Gyrate atrophy, especially children, who are undergoing arginine restriction in their diet with low total protein intake should receive enough calories in their diet supplemented by essential amino acids, vitamins, and minerals to avoid malnutrition and excessive break down of their endogenous proteins.[37]
Staging
Four stages of gyrate atrophy have been described according to the disease progression [1]:
- Stage I is characterized by sharply defined and separate areas of peripheral chorioretinal atrophy with a normal disc, macula, and retinal vasculature, and concentric visual field limitation.
- Stage II is characterized by sharp areas of chorioretinal atrophy that become more fused and spread towards the posterior pole with narrower vessels, peripapillary degeneration, and similar concentric field limitation as stage I.
- Stage III is characterized by a large area of peripapillary degeneration, with an annular zone of a functioning retina between it and the peripheral degeneration, and a pale optic disc with narrower vessels. The macula is still spared with fine pigmentation, but the visual field is more deteriorated.
- Stage IV is characterized by a posterior pole that is completely atrophic with only a small functioning macular area and an extremely narrow retinal vasculature. Some patients also developed pigmentation with peripheral crystals.
Prognosis
Patients with GA usually present with progressive night blindness and visual field constriction that starts in the first decade due to progressive chorioretinal degeneration, which can usually be slowed down by arginine restriction in their diet and vitamin B6 supplementation occasionally leading to a picture similar to early retinitis pigmentosa.[5][55] This is followed by a diminution of the central visual acuity, which usually occurs in the first or second decade due to progressive macular changes and cataract formation, which can be managed by treatment of the macular conditions and cataract extraction, respectively, leading to visual acuity improvement.[5][6] Irreversible loss of vision and blindness (vision less than 20/200) occurs when the chorioretinal atrophy reaches the central macular area, which usually occurs between 40 and 55 years of age.[5] This, however, can be variable depending on several factors, including treatment.[5][55] The development of certain complications associated with GA such as macular holes, subfoveal choroidal neovascularization, and retinal detachment can lead to an earlier and more severe loss of vision.
Complications
- Macular complications
- Vitreoretinal complications
- Neurological complications
Deterrence and Patient Education
Patients and their parents must be educated about the condition and the importance of long-term compliance with dietary modifications, including the restriction of arginine in their diet since this has been shown to influence the visual function of the patients greatly. Screening of family members of patients is also very important to allow dietary measures to be implemented at an early age since this was also shown to influence the visual outcome greatly.[55]
Enhancing Healthcare Team Outcomes
The proper management of gyrate atrophy of the choroid and retina requires the combined efforts of pediatricians, ophthalmologists, neurologists, geneticists, dieticians, and other health professionals. Only effective interprofessional communication between these health care providers across a wide range of disciplines, and with patients and their parents, can ensure that proper care is delivered to these patients. The currently most effective approach in the management of GA is the early diagnosis of the condition followed by dietary modifications, specifically the restriction of arginine in the diet, and treatment of complications.[55] This can be achieved by the early screening and referral of patients followed by proper patient education, evidence-based management, and long-term follow up. Currently, however, most of the evidence regarding the treatment of this rare autosomal recessive condition and its complications comes from historical cohort studies and case series. [Level 4 and 5]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Takki K. Gyrate atrophy of the choroid and retina associated with hyperornithinaemia. The British journal of ophthalmology. 1974 Jan:58(1):3-23 [PubMed PMID: 4841281]
Simell O, Takki K. Raised plasma-ornithine and gyrate atrophy of the choroid and retina. Lancet (London, England). 1973 May 12:1(7811):1031-3 [PubMed PMID: 4122112]
Kaiser-Kupfer MI, Valle D, Del Valle LA. A specific enzyme defect in gyrate atrophy. American journal of ophthalmology. 1978 Feb:85(2):200-4 [PubMed PMID: 623190]
Level 3 (low-level) evidenceMichaud J, Brody LC, Steel G, Fontaine G, Martin LS, Valle D, Mitchell G. Strand-separating conformational polymorphism analysis: efficacy of detection of point mutations in the human ornithine delta-aminotransferase gene. Genomics. 1992 Jun:13(2):389-94 [PubMed PMID: 1612597]
Takki KK, Milton RC. The natural history of gyrate atrophy of the choroid and retina. Ophthalmology. 1981 Apr:88(4):292-301 [PubMed PMID: 7254775]
Elnahry AG, Hassan FK, Abdel-Kader AA. Bevacizumab for the treatment of intraretinal cystic spaces in a patient with gyrate atrophy of the choroid and retina. Ophthalmic genetics. 2018 Dec:39(6):759-762. doi: 10.1080/13816810.2018.1536220. Epub 2018 Oct 18 [PubMed PMID: 30335551]
Hayasaka S, Shiono T, Mizuno K, Sasayama C, Akiya S, Tanaka Y, Hayakawa M, Miyake Y, Ohba N. Gyrate atrophy of the choroid and retina: 15 Japanese patients. The British journal of ophthalmology. 1986 Aug:70(8):612-4 [PubMed PMID: 3741829]
Bargum R. Differential diagnosis of normoornithinaemic gyrate atrophy of the choroid and retina. Acta ophthalmologica. 1986 Aug:64(4):369-73 [PubMed PMID: 3776498]
Level 3 (low-level) evidenceKaiser-Kupfer MI, Caruso RC, Valle D. Gyrate atrophy of the choroid and retina. Long-term reduction of ornithine slows retinal degeneration. Archives of ophthalmology (Chicago, Ill. : 1960). 1991 Nov:109(11):1539-48 [PubMed PMID: 1755734]
Level 3 (low-level) evidenceWeleber RG, Kennaway NG. Clinical trial of vitamin B6 for gyrate atrophy of the choroid and retina. Ophthalmology. 1981 Apr:88(4):316-24 [PubMed PMID: 6789268]
Kaiser-Kupfer MI, de Monasterio F, Valle D, Walser M, Brusilow S. Visual results of a long-term trial of a low-arginine diet in gyrate atrophy of choroid and retina. Ophthalmology. 1981 Apr:88(4):307-10 [PubMed PMID: 6973117]
Level 3 (low-level) evidenceValle D, Kaiser-Kupfer MI, Del Valle LA. Gyrate atrophy of the choroid and retina: deficiency of ornithine aminotransferase in transformed lymphocytes. Proceedings of the National Academy of Sciences of the United States of America. 1977 Nov:74(11):5159-61 [PubMed PMID: 270753]
Level 3 (low-level) evidenceBerson EL. Nutrition and retinal degenerations. International ophthalmology clinics. 2000 Fall:40(4):93-111 [PubMed PMID: 11064860]
Brody LC, Mitchell GA, Obie C, Michaud J, Steel G, Fontaine G, Robert MF, Sipila I, Kaiser-Kupfer M, Valle D. Ornithine delta-aminotransferase mutations in gyrate atrophy. Allelic heterogeneity and functional consequences. The Journal of biological chemistry. 1992 Feb 15:267(5):3302-7 [PubMed PMID: 1737786]
Level 3 (low-level) evidenceWang T, Steel G, Milam AH, Valle D. Correction of ornithine accumulation prevents retinal degeneration in a mouse model of gyrate atrophy of the choroid and retina. Proceedings of the National Academy of Sciences of the United States of America. 2000 Feb 1:97(3):1224-9 [PubMed PMID: 10655512]
Level 3 (low-level) evidenceKhan MY, Ibraheim AS, Firoozmand S. Gyrate atrophy of the choroid and retina with hyperornithinaemia, cystinuria and lysinuria. Eye (London, England). 1994:8 ( Pt 3)():284-7 [PubMed PMID: 7958031]
Level 3 (low-level) evidenceValle D, Walser M, Brusilow S, Kaiser-Kupfer MI, Takki K. Gyrate atrophy of the choroid and retina. Biochemical considerations and experience with an arginine-restricted diet. Ophthalmology. 1981 Apr:88(4):325-30 [PubMed PMID: 7254778]
De Jonge WJ, Dingemanse MA, de Boer PA, Lamers WH, Moorman AF. Arginine-metabolizing enzymes in the developing rat small intestine. Pediatric research. 1998 Apr:43(4 Pt 1):442-51 [PubMed PMID: 9544996]
Level 3 (low-level) evidenceWhitcup SM, Iwata F, Podgor MJ, Valle D, Sran PK, Kaiser-Kupfer MI. Association of thyroid disease with retinitis pigmentosa and gyrate atrophy. American journal of ophthalmology. 1996 Dec:122(6):903-5 [PubMed PMID: 8956655]
Level 2 (mid-level) evidenceRenner AB, Walter A, Fiebig BS, Jägle H. Gyrate atrophy: clinical and genetic findings in a female without arginine-restricted diet during her first 39 years of life and report of a new OAT gene mutation. Documenta ophthalmologica. Advances in ophthalmology. 2012 Aug:125(1):81-9. doi: 10.1007/s10633-012-9335-0. Epub 2012 Jun 7 [PubMed PMID: 22674428]
Level 3 (low-level) evidenceJasani KM, Parry NRA, Black G, Kelly SP. Unique case of gyrate atrophy with a well-preserved electroretinogram (ERG). BMJ case reports. 2018 Feb 5:2018():. pii: bcr-2016-217556. doi: 10.1136/bcr-2016-217556. Epub 2018 Feb 5 [PubMed PMID: 29437727]
Level 3 (low-level) evidenceTripathy K, Chawla R, Sharma YR, Gogia V. Ultrawide field fluorescein angiogram in a family with gyrate atrophy and foveoschisis. Oman journal of ophthalmology. 2016 May-Aug:9(2):104-6. doi: 10.4103/0974-620X.184529. Epub [PubMed PMID: 27433038]
Huang J, Fu J, Fu S, Yang L, Nie K, Duan C, Cheng J, Li Y, Lv H, Chen R, Liu L, Fu J. Diagnostic value of a combination of next-generation sequencing, chorioretinal imaging and metabolic analysis: lessons from a consanguineous Chinese family with gyrate atrophy of the choroid and retina stemming from a novel OAT variant. The British journal of ophthalmology. 2019 Mar:103(3):428-435. doi: 10.1136/bjophthalmol-2018-312347. Epub 2018 Oct 26 [PubMed PMID: 30366948]
Moloney TP, O'Hagan S, Lee L. Ultrawide-field fundus photography of the first reported case of gyrate atrophy from Australia. Clinical ophthalmology (Auckland, N.Z.). 2014:8():1561-3. doi: 10.2147/OPTH.S64248. Epub 2014 Aug 20 [PubMed PMID: 25187693]
Level 3 (low-level) evidenceAngioï-Duprez K, Maalouf T, George JL. [Gyrate atrophy and craniopharyngioma: a case report]. Journal francais d'ophtalmologie. 2001 May:24(5):513-6 [PubMed PMID: 11397989]
Level 3 (low-level) evidenceZhioua Braham I, Ammous I, Maalej R, Boukari M, Mili Boussen I, Errais K, Zhioua R. Multimodal imaging of foveoschisis and macular pseudohole associated with gyrate atrophy: a family report. BMC ophthalmology. 2018 Apr 12:18(1):89. doi: 10.1186/s12886-018-0755-9. Epub 2018 Apr 12 [PubMed PMID: 29649987]
Kim SJ, Lim DH, Kim JH, Kang SW. Gyrate atrophy of the choroid and retina diagnosed by ornithine-δ-aminotransferase gene analysis: a case report. Korean journal of ophthalmology : KJO. 2013 Oct:27(5):388-91. doi: 10.3341/kjo.2013.27.5.388. Epub 2013 Sep 10 [PubMed PMID: 24082780]
Level 3 (low-level) evidenceVasconcelos-Santos DV, Magalhães EP, Nehemy MB. Macular edema associated with gyrate atrophy managed with intravitreal triamcinolone: a case report. Arquivos brasileiros de oftalmologia. 2007 Sep-Oct:70(5):858-61 [PubMed PMID: 18157315]
Level 3 (low-level) evidenceShrestha SP, Arora R, Pradhan R, Bhatt S. First reported cases of gyrate atrophy of the choroid from Nepal. BMJ case reports. 2010 Nov 29:2010():. doi: 10.1136/bcr.04.2010.2951. Epub 2010 Nov 29 [PubMed PMID: 22798087]
Level 3 (low-level) evidenceAlparslan Ş, Fatih MT, Muhammed Ş, Adnan Y. Cystoid macular edema secondary to gyrate atrophy in a child treated with sub-tenon injection of triamcinolone acetonide. Romanian journal of ophthalmology. 2018 Jul-Sep:62(3):246-249 [PubMed PMID: 30505995]
Mäntyjärvi M, Tuppurainen K. Colour vision in gyrate atrophy. Vision research. 1998 Nov:38(21):3409-12 [PubMed PMID: 9893857]
Level 3 (low-level) evidenceKaiser-Kupfer MI, Valle D, Bron AJ. Clinical and biochemical heterogeneity in gyrate atrophy. American journal of ophthalmology. 1980 Feb:89(2):219-22 [PubMed PMID: 7355975]
Level 3 (low-level) evidenceWang T, Milam AH, Steel G, Valle D. A mouse model of gyrate atrophy of the choroid and retina. Early retinal pigment epithelium damage and progressive retinal degeneration. The Journal of clinical investigation. 1996 Jun 15:97(12):2753-62 [PubMed PMID: 8675686]
Level 3 (low-level) evidenceRatzlaff K, Baich A. Comparison of ornithine aminotransferase activities in the pigment epithelium and retina of vertebrates. Comparative biochemistry and physiology. B, Comparative biochemistry. 1987:88(1):35-7 [PubMed PMID: 3677612]
Level 3 (low-level) evidenceRao GN, Cotlier E. Ornithine delta-aminotransferase activity in retina and other tissues. Neurochemical research. 1984 Apr:9(4):555-62 [PubMed PMID: 6462326]
Level 3 (low-level) evidenceKorte GE, Reppucci V, Henkind P. RPE destruction causes choriocapillary atrophy. Investigative ophthalmology & visual science. 1984 Oct:25(10):1135-45 [PubMed PMID: 6480292]
Level 3 (low-level) evidenceValle D, Walser M, Brusilow SW, Kaiser-Kupfer M. Gyrate atrophy of the choroid and retina: amino acid metabolism and correction of hyperornithinemia with an arginine-deficient diet. The Journal of clinical investigation. 1980 Feb:65(2):371-8 [PubMed PMID: 7356686]
Valtonen M, Näntö-Salonen K, Jääskeläinen S, Heinänen K, Alanen A, Heinonen OJ, Lundbom N, Erkintalo M, Simell O. Central nervous system involvement in gyrate atrophy of the choroid and retina with hyperornithinaemia. Journal of inherited metabolic disease. 1999 Dec:22(8):855-66 [PubMed PMID: 10604138]
Sipilä I, Simell O, Rapola J, Sainio K, Tuuteri L. Gyrate atrophy of the choroid and retina with hyperornithinemia: tubular aggregates and type 2 fiber atrophy in muscle. Neurology. 1979 Jul:29(7):996-1005 [PubMed PMID: 572946]
Wilson DJ, Weleber RG, Green WR. Ocular clinicopathologic study of gyrate atrophy. American journal of ophthalmology. 1991 Jan 15:111(1):24-33 [PubMed PMID: 1985486]
Level 3 (low-level) evidenceValle DL, Boison AP, Jezyk P, Aguirre G. Gyrate atrophy of the choroid and retina in a cat. Investigative ophthalmology & visual science. 1981 Feb:20(2):251-5 [PubMed PMID: 7461927]
Level 3 (low-level) evidenceValtonen M, Näntö-Salonen K, Heinänen K, Alanen A, Kalimo H, Simell O. Skeletal muscle of patients with gyrate atrophy of the choroid and retina and hyperornithinaemia in ultralow-field magnetic resonance imaging and computed tomography. Journal of inherited metabolic disease. 1996:19(6):729-34 [PubMed PMID: 8982944]
Oliveira TL, Andrade RE, Muccioli C, Sallum J, Belfort R Jr. Cystoid macular edema in gyrate atrophy of the choroid and retina: a fluorescein angiography and optical coherence tomography evaluation. American journal of ophthalmology. 2005 Jul:140(1):147-9 [PubMed PMID: 16038665]
Level 3 (low-level) evidenceParameswarappa DC, Agarwal K. Bilateral macular hole in gyrate atrophy: A rare association. Indian journal of ophthalmology. 2020 Apr:68(4):652. doi: 10.4103/ijo.IJO_984_19. Epub [PubMed PMID: 32174595]
Inanc M, Tekin K, Teke MY. Bilateral choroidal neovascularization associated with gyrate atrophy managed with intravitreal bevacizumab. International ophthalmology. 2018 Jun:38(3):1351-1355. doi: 10.1007/s10792-017-0579-2. Epub 2017 May 30 [PubMed PMID: 28560651]
Peltola KE, Jääskeläinen S, Heinonen OJ, Falck B, Näntö-Salonen K, Heinänen K, Simell O. Peripheral nervous system in gyrate atrophy of the choroid and retina with hyperornithinemia. Neurology. 2002 Sep 10:59(5):735-40 [PubMed PMID: 12221166]
Kennaway NG, Stankova L, Wirtz MK, Weleber RG. Gyrate atrophy of the choroid and retina: characterization of mutant ornithine aminotransferase and mechanism of response to vitamin B6. American journal of human genetics. 1989 Mar:44(3):344-52 [PubMed PMID: 2916580]
Valayannopoulos V, Boddaert N, Mention K, Touati G, Barbier V, Chabli A, Sedel F, Kaplan J, Dufier JL, Seidenwurm D, Rabier D, Saudubray JM, de Lonlay P. Secondary creatine deficiency in ornithine delta-aminotransferase deficiency. Molecular genetics and metabolism. 2009 Jun:97(2):109-13. doi: 10.1016/j.ymgme.2008.12.010. Epub 2009 Mar 31 [PubMed PMID: 19345633]
Level 2 (mid-level) evidenceVannas-Sulonen K. Progression of gyrate atrophy of the choroid and retina. A long-term follow-up by fluorescein angiography. Acta ophthalmologica. 1987 Feb:65(1):101-9 [PubMed PMID: 3577698]
Chatziralli I, Theodossiadis G, Emfietzoglou I, Theodossiadis P. Intravitreal ranibizumab for choroidal neovascularization secondary to gyrate atrophy in a young patient: a multimodal imaging analysis. European journal of ophthalmology. 2015 Oct 21:25(6):e119-22. doi: 10.5301/ejo.5000660. Epub 2015 Oct 21 [PubMed PMID: 26419008]
Marano F, Deutman AF, Pinckers AJ, Aandekerk AL. Gyrate atrophy and choroidal neovascularization. Archives of ophthalmology (Chicago, Ill. : 1960). 1996 Oct:114(10):1295 [PubMed PMID: 8859105]
Level 3 (low-level) evidenceSalcedo-Villanueva G, Paciuc-Beja M, Villanueva-Mendoza C, Harasawa M, Smith JM, Velez-Montoya R, Olson JL, Oliver SC, Mandava N, Quiroz-Mercado H. Progression of gyrate atrophy measured with ultra-wide-field imaging. International ophthalmology. 2016 Feb:36(1):111-120. doi: 10.1007/s10792-015-0085-3. Epub 2015 May 26 [PubMed PMID: 26003990]
Raitta C, Carlson S, Vannas-Sulonen K. Gyrate atrophy of the choroid and retina: ERG of the neural retina and the pigment epithelium. The British journal of ophthalmology. 1990 Jun:74(6):363-7 [PubMed PMID: 2378845]
Weleber RG, Kennaway NG, Buist NR. Gyrate atrophy of the choroid and retina. Approaches to therapy. International ophthalmology. 1981 Aug:4(1-2):23-32 [PubMed PMID: 7028650]
Kaiser-Kupfer MI, Caruso RC, Valle D. Gyrate atrophy of the choroid and retina: further experience with long-term reduction of ornithine levels in children. Archives of ophthalmology (Chicago, Ill. : 1960). 2002 Feb:120(2):146-53 [PubMed PMID: 11831916]
Level 3 (low-level) evidenceSergouniotis PI, Davidson AE, Lenassi E, Devery SR, Moore AT, Webster AR. Retinal structure, function, and molecular pathologic features in gyrate atrophy. Ophthalmology. 2012 Mar:119(3):596-605. doi: 10.1016/j.ophtha.2011.09.017. Epub 2011 Dec 17 [PubMed PMID: 22182799]
Level 2 (mid-level) evidenceMansour AM, Elnahry AG, Tripathy K, Foster RE, Mehanna CJ, Vishal R, Çavdarlı C, Arrigo A, Parodi MB. Analysis of optical coherence angiography in cystoid macular oedema associated with gyrate atrophy. Eye (London, England). 2021 Jun:35(6):1766-1774. doi: 10.1038/s41433-020-01166-6. Epub 2020 Sep 1 [PubMed PMID: 32873946]
Raval V, Kapoor A, Nayak S, Rao S, Das T. Optical Coherence Tomography Angiography and Macular Vessel Density Analysis of Cystoid Macular Edema in Gyrate Atrophy. Ophthalmic surgery, lasers & imaging retina. 2019 Jul 1:50(7):423-427. doi: 10.3928/23258160-20190703-03. Epub [PubMed PMID: 31344241]
McInnes RR, Arshinoff SA, Bell L, McCulloch C. Treatment of gyrate atrophy of the choroid and retina with low arginine diet. Transactions of the American Ophthalmological Society. 1980:78():226-42 [PubMed PMID: 7257057]
Level 3 (low-level) evidenceMashima YG, Weleber RG, Kennaway NG, Inana G. Genotype-phenotype correlation of a pyridoxine-responsive form of gyrate atrophy. Ophthalmic genetics. 1999 Dec:20(4):219-24 [PubMed PMID: 10617919]
Santinelli R, Costagliola C, Tolone C, D'Aloia A, D'Avanzo A, Prisco F, Perrone L, del Giudice EM. Low-protein diet and progression of retinal degeneration in gyrate atrophy of the choroid and retina: a twenty-six-year follow-up. Journal of inherited metabolic disease. 2004:27(2):187-96 [PubMed PMID: 15159649]
Level 3 (low-level) evidenceVannas-Sulonen K, Simell O, Sipilä I. Gyrate atrophy of the choroid and retina. The ocular disease progresses in juvenile patients despite normal or near normal plasma ornithine concentration. Ophthalmology. 1987 Nov:94(11):1428-33 [PubMed PMID: 3684217]
Ros E. Nuts and CVD. The British journal of nutrition. 2015 Apr:113 Suppl 2():S111-20. doi: 10.1017/S0007114514003924. Epub [PubMed PMID: 26148914]
Michaud J, Thompson GN, Brody LC, Steel G, Obie C, Fontaine G, Schappert K, Keith CG, Valle D, Mitchell GA. Pyridoxine-responsive gyrate atrophy of the choroid and retina: clinical and biochemical correlates of the mutation A226V. American journal of human genetics. 1995 Mar:56(3):616-22 [PubMed PMID: 7887415]
Level 3 (low-level) evidenceHayasaka S, Saito T, Nakajima H, Takahashi O, Mizuno K, Tada K. Clinical trials of vitamin B6 and proline supplementation for gyrate atrophy of the choroid and retina. The British journal of ophthalmology. 1985 Apr:69(4):283-90 [PubMed PMID: 3922397]
Level 3 (low-level) evidenceJavadzadeh A, Gharabaghi D. Gyrate atrophy of the choroid and retina with hyper-ornithinemia responsive to vitamin B6: a case report. Journal of medical case reports. 2007 Jun 12:1():27 [PubMed PMID: 17565677]
Level 3 (low-level) evidenceSipilä I, Rapola J, Simell O, Vannas A. Supplementary creatine as a treatment for gyrate atrophy of the choroid and retina. The New England journal of medicine. 1981 Apr 9:304(15):867-70 [PubMed PMID: 7207523]
Vannas-Sulonen K, Sipilä I, Vannas A, Simell O, Rapola J. Gyrate atrophy of the choroid and retina. A five-year follow-up of creatine supplementation. Ophthalmology. 1985 Dec:92(12):1719-27 [PubMed PMID: 4088625]
Casalino G, Pierro L, Manitto MP, Michaelides M, Bandello F. Resolution of cystoid macular edema following arginine-restricted diet and vitamin B6 supplementation in a case of gyrate atrophy. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus. 2018 Aug:22(4):321-323. doi: 10.1016/j.jaapos.2017.12.016. Epub 2018 Apr 12 [PubMed PMID: 29654911]
Level 3 (low-level) evidenceÇavdarlı C, Şahlı E, Çavdarlı B, Alp MN. Regression of macular edema with topical brinzolamide and nepafenac alone and identification of a novel gyrate atrophy mutation. Arquivos brasileiros de oftalmologia. 2020 Mar-Apr:83(2):149-152. doi: 10.5935/0004-2749.20200028. Epub [PubMed PMID: 32159596]
Piozzi E, Alessi S, Santambrogio S, Cillino G, Mazza M, Iggui A, Cillino S. Carbonic Anhydrase Inhibitor with Topical NSAID Therapy to Manage Cystoid Macular Edema in a Case of Gyrate Atrophy. European journal of ophthalmology. 2017 Nov 8:27(6):e179-e183. doi: 10.5301/ejo.5001010. Epub [PubMed PMID: 28708224]
Level 3 (low-level) evidenceElnahry AG. Letter to the editor regarding: "cystoid macular edema secondary to gyrate atrophy in a child treated with sub-tenon injection of triamcinolone acetonide". Romanian journal of ophthalmology. 2018 Oct-Dec:62(4):317-318 [PubMed PMID: 30891531]
Level 3 (low-level) evidenceAbdelmassih Y, El-Khoury S, Cherfan CG. Dexamethasone implant for the treatment of gyrate atrophy associated macular edema. Journal francais d'ophtalmologie. 2019 Jan:42(1):e1-e4. doi: 10.1016/j.jfo.2018.03.029. Epub 2018 Dec 14 [PubMed PMID: 30559013]
Elnahry AG, Aboulfotouh MR, Nassar GA. Treatment of Intraretinal Cystic Spaces Associated With Gyrate Atrophy of the Choroid and Retina With Intravitreal Bevacizumab. Journal of pediatric ophthalmology and strabismus. 2020 Nov 1:57(6):400-406. doi: 10.3928/01913913-20200813-01. Epub [PubMed PMID: 33211898]
Tsilou E, Rubin BI, Abraham FA, Kaiser-Kupfer M. Bilateral late posterior chamber intraocular lens dislocation with the capsular bag in a patient with gyrate atrophy. Journal of cataract and refractive surgery. 2004 Jul:30(7):1593-4 [PubMed PMID: 15210246]
Level 3 (low-level) evidenceBerbel RF, Rauen PI, Pallone RF, Casella AM. Retinal detachment and gyrate atrophy of the choroid and retina: case report. Arquivos brasileiros de oftalmologia. 2012 Jan-Feb:75(1):59-60 [PubMed PMID: 22552420]
Level 3 (low-level) evidenceTakahashi O, Hayasaka S, Kiyosawa M, Mizuno K, Saito T, Tada K, Igarashi Y. Gyrate atrophy of choroid and retina complicated by vitreous hemorrhage. Japanese journal of ophthalmology. 1985:29(2):170-6 [PubMed PMID: 4046225]
Level 3 (low-level) evidenceDimopoulos IS, Radziwon A, St Laurent CD, MacDonald IM. Choroideremia. Current opinion in ophthalmology. 2017 Sep:28(5):410-415. doi: 10.1097/ICU.0000000000000392. Epub [PubMed PMID: 28520608]
Level 3 (low-level) evidenceVerbakel SK, van Huet RAC, Boon CJF, den Hollander AI, Collin RWJ, Klaver CCW, Hoyng CB, Roepman R, Klevering BJ. Non-syndromic retinitis pigmentosa. Progress in retinal and eye research. 2018 Sep:66():157-186. doi: 10.1016/j.preteyeres.2018.03.005. Epub 2018 Mar 27 [PubMed PMID: 29597005]
Zeitz C, Robson AG, Audo I. Congenital stationary night blindness: an analysis and update of genotype-phenotype correlations and pathogenic mechanisms. Progress in retinal and eye research. 2015 Mar:45():58-110. doi: 10.1016/j.preteyeres.2014.09.001. Epub 2014 Oct 13 [PubMed PMID: 25307992]
Level 3 (low-level) evidenceMolday RS, Kellner U, Weber BH. X-linked juvenile retinoschisis: clinical diagnosis, genetic analysis, and molecular mechanisms. Progress in retinal and eye research. 2012 May:31(3):195-212. doi: 10.1016/j.preteyeres.2011.12.002. Epub 2012 Jan 3 [PubMed PMID: 22245536]
Level 3 (low-level) evidenceGarcía-García GP, Martínez-Rubio M, Moya-Moya MA, Pérez-Santonja JJ, Escribano J. Current perspectives in Bietti crystalline dystrophy. Clinical ophthalmology (Auckland, N.Z.). 2019:13():1379-1399. doi: 10.2147/OPTH.S185744. Epub 2019 Jul 30 [PubMed PMID: 31440027]
Level 3 (low-level) evidenceTsang SH, Sharma T. Pigmented Paravenous Chorioretinal Atrophy (PPCRA). Advances in experimental medicine and biology. 2018:1085():111-113. doi: 10.1007/978-3-319-95046-4_22. Epub [PubMed PMID: 30578495]
Level 3 (low-level) evidenceGodley BF, Tiffin PA, Evans K, Kelsell RE, Hunt DM, Bird AC. Clinical features of progressive bifocal chorioretinal atrophy: a retinal dystrophy linked to chromosome 6q. Ophthalmology. 1996 Jun:103(6):893-8 [PubMed PMID: 8643244]
Elnahry AG, Khafagy MM, Esmat SM, Mortada HA. Prevalence and Associations of Posterior Segment Manifestations in a Cohort of Egyptian Patients with Pathological Myopia. Current eye research. 2019 Sep:44(9):955-962. doi: 10.1080/02713683.2019.1606252. Epub 2019 Apr 30 [PubMed PMID: 30964360]
Hwang JC, Kim DY, Chou CL, Tsang SH. Fundus autofluorescence, optical coherence tomography, and electroretinogram findings in choroidal sclerosis. Retina (Philadelphia, Pa.). 2010 Jul-Aug:30(7):1095-103. doi: 10.1097/IAE.0b013e3181cd48f9. Epub [PubMed PMID: 20224472]
Level 3 (low-level) evidenceKellner U, Weleber RG, Kennaway NG, Fishman GA, Foerster MH. Gyrate atrophy-like phenotype with normal plasma ornithine. Retina (Philadelphia, Pa.). 1997:17(5):403-13 [PubMed PMID: 9355188]
Level 3 (low-level) evidenceLabiano AT, Arroyo MH. Gyrate atrophy-like phenotype with normal plasma ornithine and low plasma taurine. GMS ophthalmology cases. 2020:10():Doc04. doi: 10.3205/oc000131. Epub 2020 Feb 27 [PubMed PMID: 32269902]
Level 3 (low-level) evidenceTripathy K, Sharma YR, Chawla R, Jain S, Behera A. Ultra-wide Field Imaging of an Operated Macular Hole in Gyrate Atrophy. Journal of ophthalmic & vision research. 2016 Jul-Sep:11(3):336-7. doi: 10.4103/2008-322X.188404. Epub [PubMed PMID: 27621797]