Introduction
Aortic valve replacement is the gold standard treatment for patients with severe or symptomatic aortic insufficiency or aortic stenosis. Historically, the conventional approach involved replacing the aortic valve through a full median sternotomy. For some patients, this midline anterior chest incision may result in inadequate healing, heightened pain, and an extended recovery period. Patients with conditions such as osteoporosis or diabetes exemplify this, as their thinned-out sternum may take an extended time to heal and be significantly painful.[1][2][3][4] Minimally invasive techniques offer improved cosmetic outcomes, quicker recovery with reduced postoperative pain, and a faster return to daily activities. These techniques have decreased intensive care unit stays and reduced hospital length of stay.[5] Minimally invasive surgical procedures require expensive disposable equipment, making them more costly than traditional surgery.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
The human heart has 4 anatomical valves crucial for regulating blood flow in and out of the myocardium. The 2 semilunar valves, pulmonic and aortic, along with the 2 atrioventricular valves, mitral and tricuspid, play essential roles. All cardiac valves, except for the mitral valve, have 3 leaflets and are surrounded by fibrous rings (annulus) for support. The aortic valve resides centrally between the ascending aorta and the left ventricular outflow tract (see Image. Aortic Valve and Image. Aortic Valve, Video). Positioned closely to other myocardial structures, this tract approximates the mitral valve posterolaterally, the pulmonic valve anteriorly, and the tricuspid valve posteromedially. The 3 sinuses of Valsalva, situated above their respective aortic valve leaflets, separate the aortic root from the left ventricle, and the sinotubular junction divides the aortic root from the ascending aorta.
While a bicuspid aortic valve occurs in 1% to 2% of the population and may lead to aortic stenosis or regurgitation, the standard trileaflet structure opens during systole, moving superiorly toward the sinuses of Valsalva. During diastole, the valve leaflets close and coapt at the level of the aortic annulus. The sinuses' design prevents interference with the ostia of the right and left coronary arteries when the leaflets are open, allowing a small blood flow space between the valve leaflets and the wall. This mechanism facilitates valve opening and closure. The aortic valve area, crucially measured during systole between the open leaflets, is a key parameter in understanding valve function.
Indications
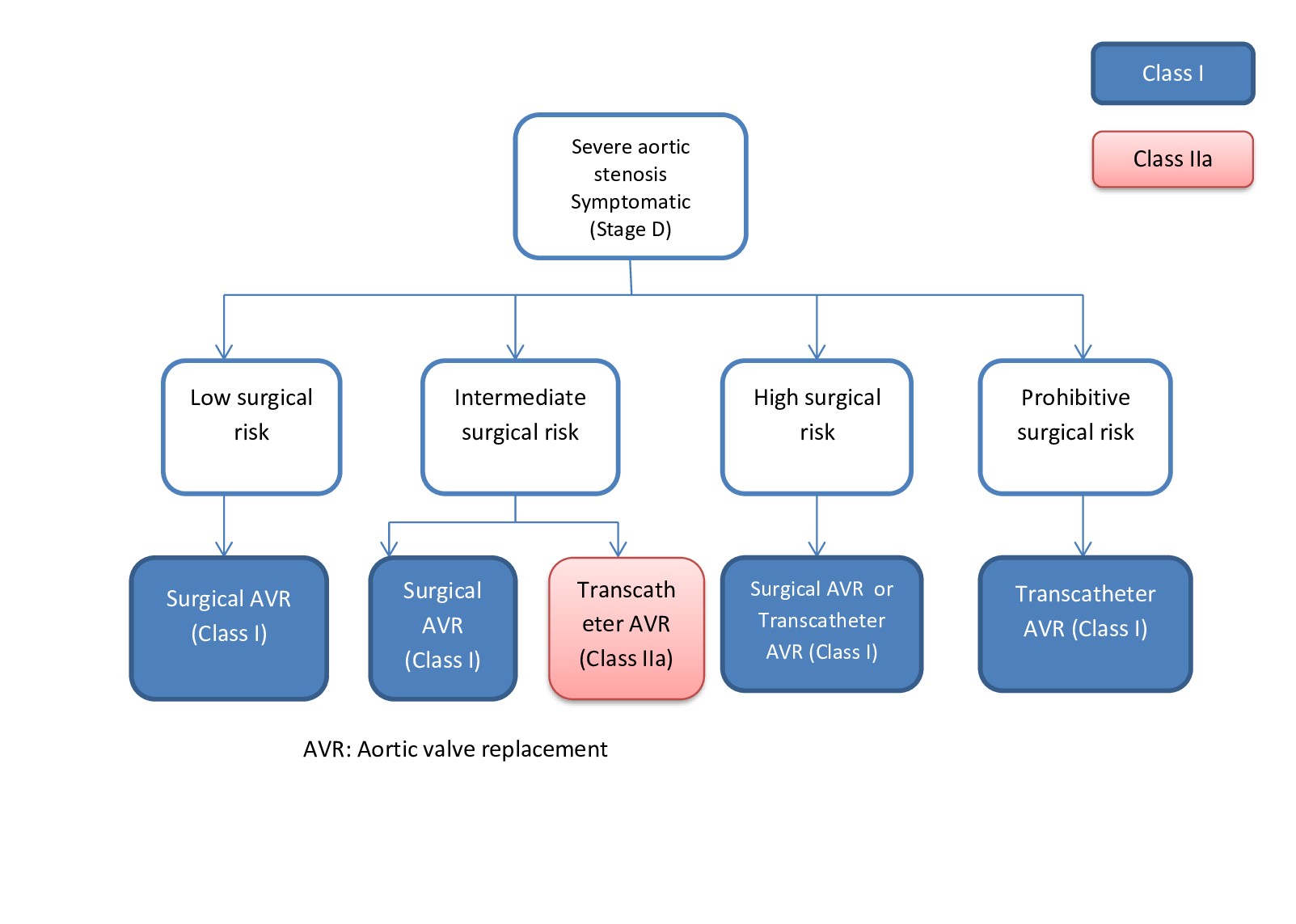
According to the 2020 American Heart Association/American College of Cardiology valve guidelines, the indications for aortic valve replacement are:
- Symptomatic severe high gradient aortic stenosis - Class 1 recommendation
- Asymptomatic severe aortic stenosis with left ventricle ejection fraction (LVEF) <50% - Class 1 recommendation
- Asymptomatic severe aortic stenosis when undergoing cardiac surgery for another reason - Class 1 recommendation
- Low flow, low gradient severe aortic stenosis with reduced LVEF - Class 1 recommendation
- Symptomatic low flow, low gradient severe aortic stenosis with normal LVEF if the aortic stenosis is the cause of the symptoms - Class 1 recommendation
- Asymptomatic patients with severe aortic stenosis and low surgical risk when exercise testing demonstrates decreased exercise tolerance of a fall in the systolic blood pressure ≥10 mm Hg from baseline - Class 2a recommendation
- Asymptomatic aortic stenosis with very severe aortic stenosis (≥5 m/s) - Class 2a recommendation
- Asymptomatic patients with severe aortic stenosis and low surgical risk when the serum B-type natriuretic peptide is >3 times normal - Class 2a recommendation
- Asymptomatic patients with high gradient severe aortic stenosis and low surgical risk when the aortic velocity increases ≥3 m/s per year - Class 2a recommendation
- Patients with severe asymptomatic aortic stenosis and a progressive decrease in LVEF (<60%) on at least 3 imaging studies - Class 2b recommendation
- Patients with moderate aortic stenosis at the time of cardiac surgery for another indication - Class 2b recommendation
Surgical aortic valve replacement is indicated for:
- Patients <65 years or with a life expectancy >20 years
- The anatomy precludes transcatheter valve placement.
- Asymptomatic patients with a class 2a indication for replacement (eg, abnormal exercise stress test, severe aortic stenosis, rapid progression, elevated B-type natriuretic peptide) (see Image. Indication for Aortic Valve Replacement).
Contraindications
The following are contraindications to minimally invasive aortic valve replacement:
- Severe chest wall deformities
- Significant dislocation of the heart and great vessels, both in lateral and posterior directions, poses challenges for safely accessing the ascending aorta and aortic valve. The associated increased chest wall rigidity accompanying such deformities further complicates exposure by restricting the opening of either the sternotomy or the thoracotomy. One example of severe chest wall deformity is pectus excavatum, with a Haller index exceeding 3.2, calculated as the maximal transverse diameter divided by the narrowest anteroposterior diameter.
- History of pneumonectomy
- Multiple authors consider a history of pneumonectomy as a contraindication for minimally invasive aortic valve replacement (MIAVR). A previous pneumonectomy can lead to a significant displacement of the heart and the great vessels, making MIAVR procedures challenging and potentially dangerous due to limited exposure. The presence of dense pleural adhesions further complicates the procedure. Patients who have undergone pneumonectomy are already at an elevated risk for perioperative and postoperative complications, and a potentially intricate and protracted MIAVR procedure heightens this risk significantly.[6]
- Severely calcified aorta
- For patients with a severely calcified ascending aorta, achieving ideal exposure of the ascending aorta and aortic arch is essential for selecting a safe location for arterial cannulation and aortic cross-clamp placement and additionally, ensuring secure closure of the aortotomy and effective bleeding control from the arterial cannulation site after decannulation relies on both optimal exposure and maximum freedom of movement. Given that exposure and freedom of movement are inherently more restricted in an MIAVR procedure, a severely calcified ascending aorta is considered a contraindication to undergoing MIAVR.
There are also a few conditions that require additional consideration when performing MIAVR:
- Previous radiation exposure to the anterior chest
- The radiotherapy causes alterations to the tissue quality of the aorta and right atrium, making the tissue more fragile. Radiation may also cause intrathoracic adhesions.
- History of pericarditis
- These patients may present extensive pericardial adhesions, posing challenges for central venous cannulation through the right atrium. Peripheral venous cannulation can overcome this difficulty; however, inserting temporary epicardial pacemaker wires may be challenging. Alternative approaches, such as temporary transjugular or transfemoral venous pacing wires, provide an alternative but require increased postoperative monitoring and potentially limit early mobilization.
Equipment
The following is a summary of the different types of aortic valves that are currently available for surgical placement:
Bioprosthetic valves (porcine or bovine):
- Stented - Perimount (Edwards), Epic (St Jude Medical), Hancock II (Medtronic)
- Stented, supraannular position - Magna Ease (Edwards), Mosaic (Medtronic)
- Stented, externally mounted leaflets - Mitroflow (Sorin), Trifecta (St. Jude Medical)
- Stentless - Freedom (Sorin), Toronto SPV (St Jude Medical), Freestyle (Medtronic), PrimaPlus (Edwards)
Mechanical valves:
- Regent (St Jude Medical) - bileaflet
- Single-tilting disc (Medtronic-Hall) - no longer used
- Caged-ball (Starr-Edwards) - historically used, original mechanical valve
- Tilting-disc (Björk-Shiley & Pfizer) - no longer used
Personnel
The following personnel are necessary for a minimally invasive aortic valve replacement procedure:
- Cardiac surgeon
- Cardiac anesthesiologist
- Operating room nurses
- Scrub nurses or techs
- Perfusionist
- Cardiac intensivists
Preparation
Preoperative
While various approaches exist for cardiac surgeons to address aortic valve issues, conventional open aortic valve replacement remains the gold standard. Despite the advantages offered by minimally invasive procedures, such techniques demand specialized expertise and involve a steeper learning curve. The potential for failure and the need for conversion to full sternotomy, with potential modifications to the cardiopulmonary bypass circuit, add complexity. Healthcare professionals managing valvular disorders should be aware of minimally invasive options. Ultimately, a comprehensive evaluation by an interprofessional team is crucial to determine the most suitable procedure for each patient, weighing the benefits and challenges associated with both open and minimally invasive approaches.[7][8] Once the decision is made that a minimally invasive approach is best for the patient, they should be counseled extensively on their disease process and the risks and benefits of surgery. Informed consent should be obtained before scheduling surgery. Preoperative assessment by anesthesia should be done on an as-needed basis.
Intraoperative
After the patient is in the operating room and positioned supinely on the bed, the induction of general endotracheal anesthesia begins. Patients should be intubated with a double-lumen endotracheal tube if single-lung ventilation is required. The patient must be sufficiently sedated and paralyzed before opening the chest to avoid tachyarrhythmias and hypertension during anesthesia. In patients with severe aortic stenosis, the surgeon must be scrubbed and ready to open the chest if an arrest occurs. Central venous access is obtained with a cordis, and a Swan-Ganz catheter is floated. A radial or femoral arterial line is usually placed as the patient is induced. The chest, abdomen, thighs, and lower extremities are prepped and draped in the usual sterile fashion.
Following the induction of anesthesia, inserting a transesophageal echo (TEE) probe permits the assessment of additional aortic or valvular pathologies. TEE also provides measurements of the annulus and identifies any potential issues that might necessitate adjustments to the surgical approach. Aortic insufficiency can be observed, informing the selection of cardioplegia strategies. Visualizing poor ejection fraction secondary to heart failure aids in preoperative planning, especially determining the plan for weaning off cardiopulmonary bypass (CPB).
Technique or Treatment
Over the past 2 decades, surgeons have developed alternative access routes to the aortic valve. The 2 main minimally invasive approaches to the aortic valve include a right anterior minithoracotomy and a ministernotomy. A third way of implanting the aortic valve is via a percutaneous approach combined with an anterior minithoracotomy, which will be described elsewhere in the discussion of transcatheter aortic valve replacement. All these approaches necessitate general anesthesia, TEE use, total CPB, and comprehensive cardiac monitoring. Preparedness for conversion to an open sternotomy is essential in case of complications, with the operating room set up similarly to conventional aortic valve surgery.
The right anterior minithoracotomy and ministernotomy approaches have demonstrated reduced postoperative pain and fewer complications compared to the standard full sternotomy method for aortic valve replacement. Both approaches are associated with a shorter hospital length of stay overall.[9][10] Further details on these techniques and others are elaborated below.
Right Anterior Minithoracotomy Approach
The patient is positioned in a supine position on the operating room table. General anesthesia is administered, and a dual lumen tube or bronchial blocker is inserted based on the anesthesia team's recommendation. A TEE is then performed. The patient is prepped and draped in the standard manner. A small incision of 4 to 5 cm is made over the third intercostal space on the right chest near the sternal edge. The muscle is dissected, and the right internal thoracic artery is divided. Once the pleura is accessed, the dissection is extended to the pericardium, creating a small pericardiotomy. An index finger is then used to examine the aorta and feel the aortic root to ensure proper alignment for optimal visualization of the aortic valve. Pericardial stay sutures are placed in a rectangular pattern around the area to secure it. Single-lung ventilation can then be initiated with increased positive end-expiratory pressure on the left lung. The ascending aorta can now be visualized and palpated, and epiaortic ultrasound is used to locate the ideal site for the aortic root vent cannulation.
After preparing the chest and exposing all relevant areas, attention is directed to the groin. A groin cutdown is performed just below the inguinal ligament, utilizing a prolene suture (4-0, 5-0, or 6-0) for pursestring placement to facilitate the insertion of arterial or venous cannulas into the femoral vessels. Full heparinization is then achieved with an appropriate activating clotting time. Under TEE guidance, wires are inserted. Arterial femoral artery cannulation is carried out using a 17 Fr or 19 Fr cannula.
In contrast, the cannula size for venous cannulation is chosen to achieve optimal flows during CPB, with a standard choice being a 25 Fr cannula. Some surgeons prefer venous cannulation first because conversion to an open sternotomy is needed to place a right atrial venous cannula if venous drainage fails. Direct aortic cannulation or a cutdown over the axillary artery can be performed in femoral arterial cannulation failure cases. A right superior pulmonary vein vent is placed to vent the left ventricle. An antegrade cardioplegia cannula is inserted into the ascending aorta since retrograde cardioplegia is not feasible due to limited right atrial exposure.
After achieving total CPB, the aorta is cross-clamped, and cardioplegia is delivered. For minimal aortic insufficiency, a full dose of antegrade cardioplegia suffices; however, for moderate or severe aortic insufficiency, direct ostial antegrade cardioplegia is required. Perfusion cools the patient to the surgeon's specified temperature (typically around 28 °C). Following adequate heart arrest, an oblique aortotomy exposes the aortic valve. Three traction sutures are placed at the valve commissure to aid in bringing the valve further into view. The aortic valve and calcium from the aortic annulus are removed. Following proper annulus exposure, the appropriately sized mechanical or bioprosthetic valve is selected. Surgeons may choose interrupted non-pledgeted 2-0 polyester sutures or pledgeted sutures for sewing the valve in place. The valve is then hand-sewn or secured with a knot-tying device to reduce bypass time. The aortotomy is closed with 4-0 prolene sutures. Right ventricular pacing wires are placed. The aortic cross-clamp is removed, and de-airing techniques are employed via the root vent. Subsequently, the right superior pulmonary vein vent is removed, and CPB is gradually weaned. The cannulas are clamped and removed once they are removed from the bypass. One to 2 small Blake drains can be inserted for chest drainage. The chest incision is meticulously closed, and the rib head is reattached to the sternum using sutures or a titanium plate if necessary.
Ministernotomy Approach
This technique is commonly employed for MIAVR, offering the advantage of a mini sternotomy with the option for easy conversion to a full sternotomy if necessary. It is particularly beneficial for re-doing aortic valve replacement and the elderly population. The technique involves a "j-shaped" sternal incision that spans the upper half of the sternum or even just the manubrium if needed. This approach ensures the essential aortic exposure required for aortic valve replacement without the extensive exposure of a traditional full sternotomy. When comparing mini sternotomy to full sternotomy outcomes, the former is associated with reduced postoperative respiratory complications, fewer blood transfusions, less postoperative pain, and shorter hospital stays. Notably, the same instruments can be used for both techniques.
The patient is positioned supine on the operating table with the customary sterile draping. A 5 to 6 cm incision is made over the upper midline, exposing the manubrium down to the right second through the fourth intercostal space. This area is dissected away from the sternum, and a "j" shaped pattern for the saw is marked using bovie cautery. While a reciprocating bone saw is an option, many prefer an oscillating one. A retractor is placed, ensuring gentle retraction to prevent injury to the right internal mammary artery; vessel ligation can be performed in case of injury or concerns. The thymic fat pad is divided and resected to enhance exposure. An inverted T-shaped incision in the pericardium provides optimal exposure, with pericardial sutures placed for excellent visibility. Standard aortic cannulation, 2-stage venous cannulation in the right atrium, right superior pulmonary vein vent placement, and an antegrade cardioplegia needle in the ascending aorta are performed. If exposure is insufficient or the j-shaped manubrial technique is used, femoral-femoral cannulation is an option. The standard-size cross-clamp is suitable, although a mini low-profile clamp may offer more space. Both bioprosthetic and mechanical valves can be placed with this technique.
Following the standard procedures for heparinization and CPB initiation, the technique described above for cross-clamping and establishing CPB is enacted at the desired cooling temperature. After cardiac arrest, the aortotomy is performed, and the steps outlined previously are followed, including placing 3 traction sutures at the valve commissure to facilitate valve exposure. The aortic valve is removed along with calcium from the aortic annulus. Once the annulus is sufficiently exposed, the valve size is determined. Surgeons may opt for interrupted non-pledgeted 2-0 polyester sutures or pledgeted sutures for valve attachment. The valve can be hand-sewn, or a knot-tying device can expedite the procedure, particularly for small aortic annulus hand-tying. The aortotomy is closed using 4-0 prolene sutures. Right ventricular pacing wires are placed, the aortic cross-clamp is removed, de-airing techniques are employed via the root vent, the right superior pulmonary vein vent is removed, and CPB is gradually weaned. Cannulas are clamped and removed upon exiting bypass, right ventricular pacing wires are placed, hemostasis is achieved, and chest tubes are inserted. Finally, the sternal incision is meticulously closed using wires.
Other Techniques
In percutaneous aortic valve surgery, a 6 cm incision is made at the left costal margin to access the heart. A TEE probe is inserted into the esophagus. Special instruments are utilized to create an opening in the apex of the left ventricle, through which a valve-containing device is guided to the aortic annulus. The stenotic valve is initially dilated. After proper placement, the valve is released at the aortic annulus, and the instruments are extracted, with closure of the ventricular hole using sutures.
Robotic-assisted valve surgery involves creating 3 to 5 small incisions, allowing the surgeon to utilize robotic arms during the procedure. The most challenging aspect of this technique is the actual anastomosis of the aortic valve. Many experts recommend combining robotic surgery with an open incision, enabling the surgeon to perform the aortic anastomosis for optimal outcomes manually.
Regardless of the chosen approach, it is imperative that, once the valve is securely seated and sutured in place, its hemodynamic performance is promptly assessed using TEE as soon as the heart can eject after coming off bypass. The proper positioning of the valve is critical to ensure it does not obstruct the native coronary vessels or impede the function of the mitral valve situated just beneath the aortic valve. Furthermore, the newly implanted valve must be firmly seated on the aortic annulus to mitigate the risk of developing a paravalvular leak or aortic insufficiency.[11][12][13]
When comparing transcatheter aortic valve replacement to surgical aortic valve placement, the left ventricular outflow gradient must remain 10 mm Hg or less. The aortic valve area should measure between 1.5 cm2 and 2.0 cm2 when a TAVR valve is implanted. TEE enables an immediate and comprehensive assessment of the newly placed valve, including its positioning, any indications of aortic insufficiency or stenosis due to left ventricular outflow tract blockage, leaflet motion, velocities, mean pressure gradients, and the effective orifice of the newly positioned valve.
Complications
Complications may include:
- Bleeding
- Heart block
- Infection
- Endocarditis
- Air embolus causing seizure or stroke
- Sudden cardiac death
- Calcific embolization with stroke
- Arrhythmias
- Valve failure
- Pneumonia
- Anesthesia complications
Clinical Significance
While minimally invasive procedures for aortic valve replacement are not yet the standard of care, their increasing prevalence yields favorable outcomes comparable to traditional full sternotomy approaches. Although complications align with standard aortic valve replacements, patients experience recovery similarly to traditional open heart surgery. Percutaneous transcatheter aortic valve replacement has undergone scrutiny in large clinical trials, demonstrating initial parity in morbidity and mortality with conventional techniques. However, more extended follow-up is crucial to ascertain these results' durability and sustained effectiveness over at least a decade.[14][15][16] The growing popularity of transcatheter aortic valve replacement adds complexity to comparisons with both minimally invasive and standard approaches, exceeding the scope of this paper.
Enhancing Healthcare Team Outcomes
Effective teamwork and communication among healthcare professionals are vital in minimally invasive aortic valve surgery to ensure patient-centered care, safety, and optimal outcomes. Surgeons and cardiologists must collaborate to determine the most suitable approach and valve selection, considering patient factors and anatomy. Intensivists play a critical role in preparing patients for surgery and managing postoperative care, while advanced practitioners assist in preoperative evaluations, surgical support, and postoperative follow-up. Nurses are responsible for perioperative care and are crucial in monitoring patients during the critical post-surgical phase. Pharmacists contribute to medication management, ensuring proper anticoagulation and pain control. Interprofessional communication is vital, ensuring seamless coordination between team members. Coordinated efforts maximize patient safety, enhance outcomes, and elevate team performance in delivering advanced, patient-centric care.
Media
(Click Image to Enlarge)
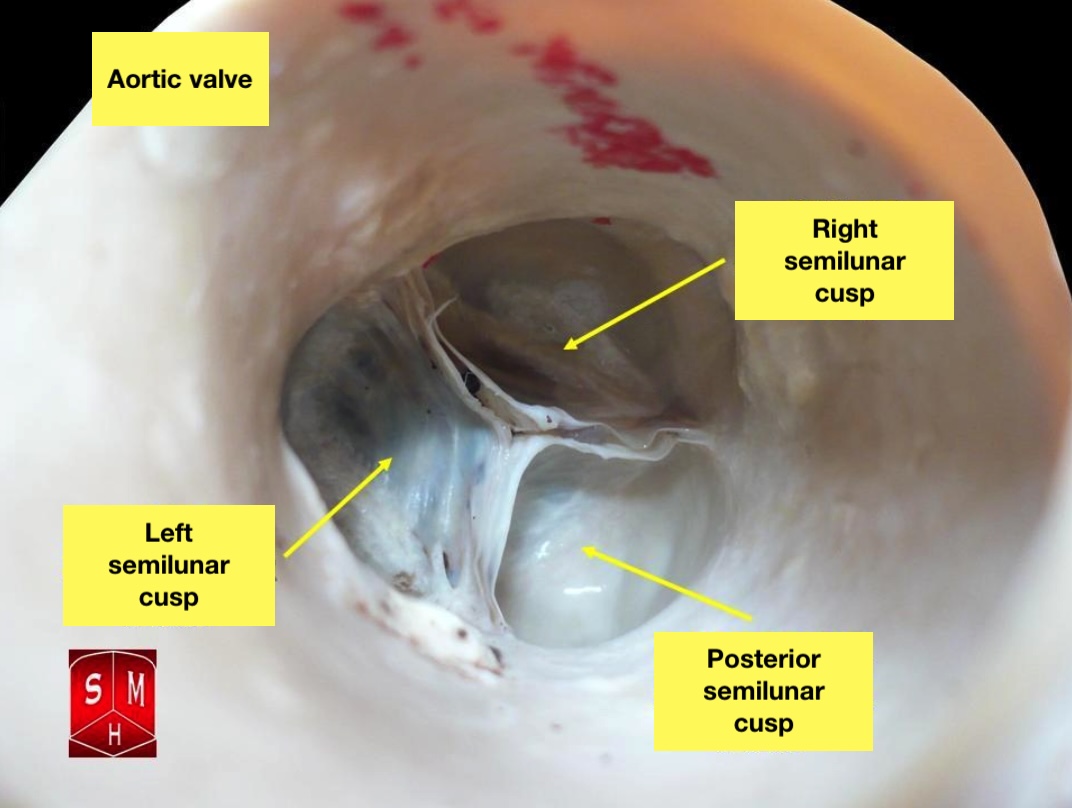
The Aortic Valve. The aortic valve resides centrally between the ascending aorta and the left ventricular outflow tract.
Anatomist90, Public Domain, via Wikimedia Commons
(Click Video to Play)
Aortic Valve Movement.
Valveguru, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
References
Ramchandani MK, Wyler von Ballmoos MC, Reardon MJ. Minimally Invasive Surgical Aortic Valve Replacement Through a Right Anterior Thoracotomy: How I Teach It. The Annals of thoracic surgery. 2019 Jan:107(1):19-23. doi: 10.1016/j.athoracsur.2018.11.006. Epub 2018 Nov 20 [PubMed PMID: 30468728]
Raptis DA, Beal MA, Kraft DC, Maniar HS, Bierhals AJ. Transcatheter Aortic Valve Replacement: Alternative Access beyond the Femoral Arterial Approach. Radiographics : a review publication of the Radiological Society of North America, Inc. 2019 Jan-Feb:39(1):30-43. doi: 10.1148/rg.2019180059. Epub 2018 Nov 23 [PubMed PMID: 30468629]
Tamadon I, Soldani G, Dario P, Menciassi A. Novel Robotic Approach for Minimally Invasive Aortic Heart Valve Surgery. Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual International Conference. 2018 Jul:2018():3656-3659. doi: 10.1109/EMBC.2018.8513309. Epub [PubMed PMID: 30441166]
Senst B, Kumar A, Diaz RR. Cardiac Surgery. StatPearls. 2024 Jan:(): [PubMed PMID: 30422530]
Khoshbin E, Prayaga S, Kinsella J, Sutherland FW. Mini-sternotomy for aortic valve replacement reduces the length of stay in the cardiac intensive care unit: meta-analysis of randomised controlled trials. BMJ open. 2011:1(2):e000266. doi: 10.1136/bmjopen-2011-000266. Epub 2011 Nov 24 [PubMed PMID: 22116090]
Level 1 (high-level) evidencevon Segesser LK, Westaby S, Pomar J, Loisance D, Groscurth P, Turina M. Less invasive aortic valve surgery: rationale and technique. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 1999 Jun:15(6):781-5 [PubMed PMID: 10431859]
Kolte D, Abbott JD, Aronow HD. Interventional Therapies for Heart Failure in Older Adults. Heart failure clinics. 2017 Jul:13(3):535-570. doi: 10.1016/j.hfc.2017.02.009. Epub [PubMed PMID: 28602371]
Yücel S, Ince H, Kische S, Sherif MA, Bushnaq H, Bärisch A, Öner A. [Interdisciplinary differential treatment of structural heart disease : When operation and when catheter-based intervention?]. Herz. 2016 Aug:41(5):443-58. doi: 10.1007/s00059-016-4444-2. Epub [PubMed PMID: 27460051]
Tamagnini G, Biondi R, Giglio MD. Aortic Valve Replacement Via Right Anterior Mini-Thoracotomy: the Conventional Procedure Performed Through a Smaller Incision. Brazilian journal of cardiovascular surgery. 2021 Feb 1:36(1):120-124. doi: 10.21470/1678-9741-2020-0165. Epub 2021 Feb 1 [PubMed PMID: 33594866]
Seitz M, Goldblatt J, Paul E, Marcus T, Larobina M, Yap CH. Minimally Invasive Aortic Valve Replacement Via Right Anterior Mini-Thoracotomy: Propensity Matched Initial Experience. Heart, lung & circulation. 2019 Feb:28(2):320-326. doi: 10.1016/j.hlc.2017.11.012. Epub 2017 Dec 7 [PubMed PMID: 29291961]
Nguyen DH, Vo AT, Le KM, Vu TT, Nguyen TT, Vu TT, Pham CVT, Truong BQ. Minimally Invasive Ozaki Procedure in Aortic Valve Disease: The Preliminary Results. Innovations (Philadelphia, Pa.). 2018 Sep/Oct:13(5):332-337. doi: 10.1097/IMI.0000000000000556. Epub [PubMed PMID: 30394956]
El-Essawi A, Breitenbach I, Haupt B, Brouwer R, Morjan M, Harringer W. Aortic valve replacement with or without myocardial revascularization in octogenarians. Can minimally invasive extracorporeal circuits improve the outcome? Perfusion. 2019 Apr:34(3):217-224. doi: 10.1177/0267659118811048. Epub 2018 Nov 3 [PubMed PMID: 30394847]
Vasanthan V, Kent W, Gregory A, Maitland A, Cutrara C, Bouchard D, Asch F, Adams C. Perceval Valve Implantation: Technical Details and Echocardiographic Assessment. The Annals of thoracic surgery. 2019 Mar:107(3):e223-e225. doi: 10.1016/j.athoracsur.2018.08.091. Epub 2018 Oct 24 [PubMed PMID: 30367839]
Rodríguez-Caulo EA, Guijarro-Contreras A, Otero-Forero J, Mataró MJ, Sánchez-Espín G, Guzón A, Porras C, Such M, Ordóñez A, Melero-Tejedor JM, Jiménez-Navarro M. Quality of life, satisfaction and outcomes after ministernotomy versus full sternotomy isolated aortic valve replacement (QUALITY-AVR): study protocol for a randomised controlled trial. Trials. 2018 Feb 17:19(1):114. doi: 10.1186/s13063-018-2486-x. Epub 2018 Feb 17 [PubMed PMID: 29454380]
Level 2 (mid-level) evidenceHerold J, Herold-Vlanti V, Sherif M, Luani B, Breyer C, Bonaventura K, Braun-Dullaeus R. Analysis of cardiovascular mortality, bleeding, vascular and cerebrovascular events in patients with atrial fibrillation vs. sinus rhythm undergoing transfemoral Transcatheter Aortic Valve Implantation (TAVR). BMC cardiovascular disorders. 2017 Dec 20:17(1):298. doi: 10.1186/s12872-017-0736-6. Epub 2017 Dec 20 [PubMed PMID: 29262768]
Oakley L, Pritchard W, Colletta J, Penny W, Romero S, Cox J, Boswell G, Kindelan J, Gramins D, Nayak K. Development and Early Experience of the First Joint Military Health System-Veterans Affairs Transcatheter Aortic Valve Replacement Program. Military medicine. 2017 Nov:182(11):e2036-e2040. doi: 10.7205/MILMED-D-16-00398. Epub [PubMed PMID: 29087877]