Introduction
Enucleation describes the removal of the entire globe, with separation of all connections from the orbit, including optic nerve transection. It is one of the oldest procedures in ophthalmology, with descriptions dating back to 2600BC.[1] The decision for enucleation can be one of the most difficult to make and discuss with the patient. The main indications for enucleation are trauma, painful eye, a blind eye, which is unsightly, intraocular malignancy, and as part of eye donation. Alternatives such as evisceration or exenteration can be considered according to the underlying diagnosis and condition of the eye.
Management of the anophthalmic socket is challenging, and a decision on implant selection and wrapping material should ideally be made as part of the pre-operative plan. Wrapping materials can be synthetic, autologous, or human tissue sourced from an eye bank. Some patients can benefit from a peg placement for improved postoperative motility and cosmesis. However, this procedure has been largely abandoned because of the occurrence of late complications, including infections, exposure, discharge, and peg loss. Ultimately, the aims of enucleation are to remove diseased tissue, improve patient comfort, replace orbital volume, and give a good functional and cosmetic result for the patient.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
A good outcome after enucleation is dependent on adequate volume replacement in orbit, the formation of fornices lined by conjunctival or mucous membranes, a well-fitting ocular prosthesis, and good cosmetic and functional eyelids.[2][3][4] Orbital fat atrophy and cicatricial orbitopathy can be due to decreased circulation, metabolism, and inadequate volume replacement, leading to features of the post-enucleation socket syndrome (PESS).[3] Damage to the lacrimal apparatus can lead to a dry anophthalmic socket or discharge and difficulties with retaining a prosthesis.[5][6]
Calculation of volume replacement with sizers is used in a subjective fashion to ascertain implant size. Adult globe size ranges from 6.9 to 9.0 mL, with axial lengths averaging between 21 mm to 29 mm.[7][8][9] Custer et al. and Kaltreider et al. introduced the concept of individualized implant size depending on the removed specimen volume or an A-scan of the contralateral eye.[8][7] Enucleation in pediatric patients can be further complicated by the need for removal of the eye when it has not reached full adult size. The eye achieves 85% of its axial length at two years of age and continues growing 1% per year until the full size is reached. Pediatric patients who undergo enucleation can require implant exchange as a secondary procedure as growth occurs.[10] Larger implants (16 to 20 mm) used at the time of primary enucleation can make secondary implants less necessary in pediatric patients.[11][12]
Indications
Before discussing enucleation with the patient, the surgeon will need to take into account their psychological needs and desires, visual potential, and anticipated complications. The main indications for enucleation are intraocular malignancy, a blind painful eye, trauma, and prevention of sympathetic ophthalmia. Other indications can be microphthalmia in a child, and phthisis.[13] Rarely, autoenucleation can take place, which is, in most cases, associated with psychosis.[14] These appear summarized below:
Intraocular Malignancy
- Choroidal melanoma
- Retinoblastoma
- Other neoplasms
Trauma
- Primary enucleation
- Secondary enucleation for sympathetic ophthalmia
- Autoenucleation (Oedipism)
Blind Painful Eye
- Neovascular glaucoma
- Endophthalmitis and uveitis
- Improve cosmesis
Contraindications
An enucleation is contraindicated in cases of intraocular malignancy with evidence of orbital spread: these patients generally require an exenteration.[15][16][17] Patients with sympathetic ophthalmia can be a relative contraindication because the sympathizing eye can ultimately be the eye with better vision, and longitudinal studies have not found a significant advantage of enucleation over evisceration for the prevention of sympathetic ophthalmia.[18][19][20] Enucleation can be relatively contraindicated in patients who will experience increased psychological trauma due to the loss of the eye.
Equipment
The equipment can vary depending on the surgical technique used for enucleation. An enucleation set should include the following surgical instruments:
- Eye speculum
- Straight dressings forceps
- Hemostatic forceps
- Straight and curved tenotomy scissors
- Enucleation scissors
- Enucleation snare
- Retractors
- Carter sphere introducer and holder
- Conformer
- Suturing set for wound closure
Personnel
Healthcare professionals required for undertaking an enucleation procedure are an ophthalmic surgeon, ophthalmic nurses, theatre scrub nurse, and medical assistants. A psychologist may be consulted before the procedure as part of the consent process, and an ocularist will be needed to produce a prosthesis as part of the patient's rehabilitation management. All children undergoing an enucleation or evisceration should be counseled by a child psychologist. Explanatory books for children and parents are also available to help make the process less traumatic.
Preparation
The surgeon should have a detailed discussion with the patient to explain the indications, risks, and benefits, as well as alternatives to enucleation. The patient will need to be aware of potential complications which are discussed in more detail under the complications section. If the patient is on nonsteroidal anti-inflammatory agents, aspirin, or anticoagulants, then, if medically possible, these can be discontinued prior to the surgery. The patient's primary care provider will need to be informed regarding any adjustment of anticoagulation medication.
Technique or Treatment
Pre-Surgery
Informed written consent is obtained from the patient, usually before the surgery date, and reconfirmed on the day, so there is adequate time to discuss any questions. The surgeon, theatre team, and patient should confirm the correct surgical site. The surgeon should personally mark the side being operated upon with the patient's consent and agreement before any medication is administered in the preoperative area. A local anesthetic injection is given as a peribulbar injection. Once the patient is under general anesthesia, it is advocated that a three-point check is performed that the correct eye is being removed: the consent form signed by the surgeon and the patient is reviewed by the surgeon, the clinical notes that record the history are reviewed by the surgeon, and the correctly marked eye is identified and agree with by the anesthesiologist, the circulating nurse, and the surgical nurse. Only then one can proceed with the procedure.
Surgical Technique
- Once a sterile field is achieved, the eyelids are retracted with the lid speculum or a traction suture.
- A retrobulbar anesthetic injection is administered with a mixture of 2% lidocaine and 0.5% marcaine with epinephrine.
- A 360-degree conjunctival peritomy is performed, and Tenon fascia is carefully removed from the globe with blunt dissection between the rectus muscles.
- Each rectus muscle is isolated with a squint hook, and the Tenon capsule is detached from the muscles.
- The muscle is secured by a locking double-armed 6-0 suture posterior to the insertions. Displacement of the muscle is prevented by clamping the suture to the surgical drape. The muscle is then transected anterior to the suture.
- The superior and inferior oblique muscles are disinserted without using tacking sutures.
- Vertical traction on the globe is created by grasping the rectus muscle insertions. The optic nerve is clamped, and enucleation scissors or snares are introduced to the posterior orbit, and the nerve is transacted. A useful technique to provide anterior traction is to use the stump of the inferior oblique.
- The optic nerve is cut using enucleation scissors.
- Careful dissection of residual fibrous attachments is undertaken with Wescott scissors.
- Minimize any orbital fat manipulation to reduce the risk of atrophy.
- Soak neurosurgical paddies/swabs in local anesthetic with epinephrine and temporarily pack these into the orbit with pressure for a few minutes. Bipolar cautery can be used to assist in hemostasis but should be used judiciously.
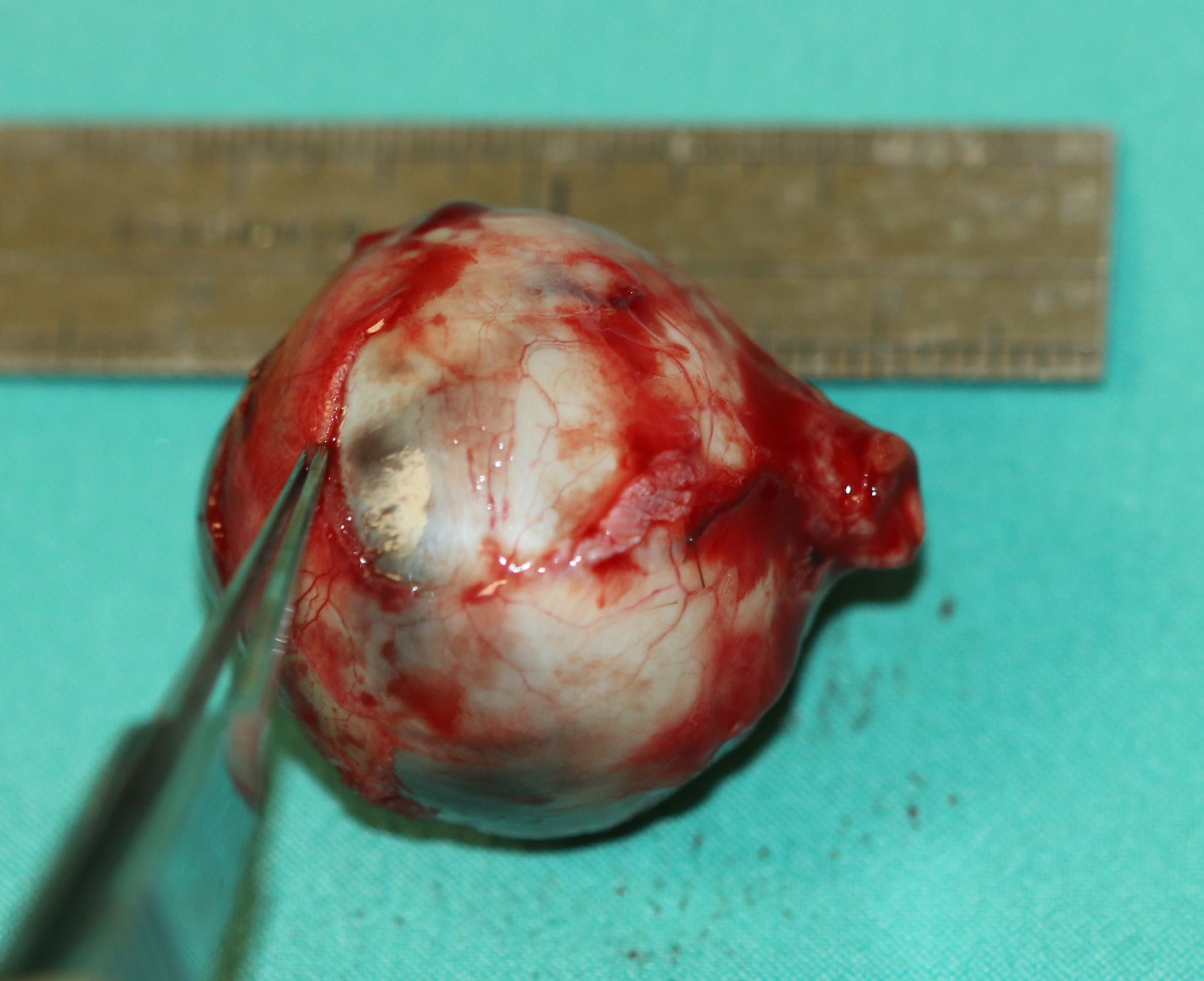
- In cases of intraocular tumors, at least 7-10 mm of the optic nerve is removed during the enucleation. It is wise to photograph the globe and the length of the optic nerve after the enucleation.
- Send the globe for pathologic investigation.
- If planning to insert the orbital implant, choose the correct size and with or without wrapping, introduce with the Carter introducer inside the muscle cone, posterior to the Tenon capsule.
- The rectus muscles are secured to the implant, and the Tenon capsule is closed with buried and interrupted 5-0 absorbable sutures.
- The conjunctiva is closed with a running 6-0 or 7-0 absorbable suture, avoiding invagination of the epithelium, which can lead to cyst formation.
- Finally, a conformer is placed to maintain the fornices and prevent symblepharon formation. Topical antibiotic ointment is applied to the fornices and lids.
- Retro-orbital injection with liposomal bupivacaine gives good postoperative pain control.[21]
- A temporary tarsorrhaphy suture is frequently inserted between the lateral, upper, and lower eyelids with a 5-0 vicryl or 6-0 vicryl suture to reduce chemosis and prevent the conformer from falling out. These sutures are removed in one to four weeks after surgery.
- The orbit is covered with a gentle pressure patch for 24 hours to reduce postoperative edema.
Post-Surgery
Oral analgesia can be given to the patient to use as required. Oral broad-spectrum antibiotic prophylaxis may be necessary in cases of infection. The pressure patch can remain in place up to a week to reduce postoperative edema, although it is generally removed in 24 hours because of the inevitable oozing and soaking of the dressing that occurs. It will need to be removed to allow the application of topical antibiotic and corticosteroid ointment onto the conformer. Patients should wash their hands prior to manipulating the conformer.
Orbital Implants
Enucleation is usually undertaken with implant insertion, either primary or as a secondary procedure. Many types of implant material have been used.[22][23][24][25][26][27][26] Porous materials include hydroxyapatite and porous polyethylene, and nonporous PMMA, silicone, and acrylic. Porous materials facilitate vascularisation, and tissue can incorporate into the implant, decreasing migration, and extrusion.
The goal of implant placement is to replace the lost volume, allow prosthesis motility, and improve symmetry with the fellow eye. Implants can be integrated, nonintegrated, buried, or exposed. The orbital contents will tend to contract towards the apex nasally and inferiorly over time, regardless of the type of implant used. This process likely leads to superior sulcus deformity. It is important to correctly size the orbital implant prior to placement. Sizers are used intraoperatively to select an implant of adequate size so that the layers of the Tenon capsule and conjunctiva can be easily apposed over its surface without tension. The addition of any wrapping material will increase the implant diameter by 1 to 2 mm and also reduce the risk of exposure of the implant.
Complications
Intraoperative
Removal of the wrong eye is the most feared intraoperative complication and can be avoided by careful attention, good communication, pre-surgery checks, and marking of the eye to be enucleated. During surgery, the loss of a rectus muscle can occur and can be minimized by placing the traction sutures carefully. If the muscle is lost and retracts into the orbit, a detailed search among the soft tissues can be undertaken. Grasping of the Tenon's fascia with forceps in a hand-over-hand fashion can help to visualize the muscle. The inter-muscular septae that are present between the extraocular muscles can be used to identify the retracted muscle. Prior to surgery, the patient's anticoagulation status will need to be ascertained and, if appropriate, discontinued during the perioperative period. Careful dissection, tissue handling, and cautery can reduce the risk of intraoperative orbital hemorrhage. Retrobulbar injection of anesthetic with epinephrine will also decrease intraoperative bleeding.
In the presence of an intraocular tumor such as melanoma or retinoblastoma, it may be necessary to obtain a long section of the optic nerve as there may be the posterior spread of the tumor along the optic nerve. When the globe is full of tumors, it can be difficult to remove an adequate length of the optic nerve (FIG 1). In these cases, usually children with large intraocular retinoblastomas, we use the superomedial approach to the orbit to identify the optic nerve and cut it under direct vision (Fig 2 & 3). This avoids the complication of transecting the globe accidentally or obtaining an inadequate length of the optic nerve.[28]
Postoperative
Early Postoperative
Postoperative orbital hemorrhage after enucleation is rare with the use of compression bandages, and the precautions discussed earlier. If severe hemorrhage occurs, surgical exploration may be necessary, and separate incisions can decrease wound dehiscence and fat atrophy. Edema of the orbit after enucleation is common and usually settles down with time. Orbital infection is a rare complication but can lead to wound dehiscence, implant exposure, and extrusion. Symptoms can be increased chemosis and persistent pain in the socket. Infection can be more common with integrated orbital implants, and treatment with systemic antibiotics can be inadequate, necessitating implant removal.[29][30] If there is dislodging of the conformer, this can result in conjunctival prolapse and fornix shortening. The patient should be advised to replace the conformer in its original position after cleaning. The temporary tarsorrhaphy sutures mentioned above help to reduce the risk of conformer extrusion.
Late Postoperative
A lax socket can develop from the secondary effects of time, gravity, and stretching of the soft tissues of the orbit by the prosthesis. It is a common late complication following enucleation, leading to downward and anterior migration of the orbital implant. A larger and heavier prosthesis can provide temporary relief but will cause greater downward migration and deepening of the superior sulcus with lower lid laxity with time.
The post-enucleation socket syndrome or anophthalmic syndrome (based upon the sterling work of Ton Smit [31]) comprises the following:
- Enophthalmos
- Deep superior sulcus
- Upper lid retraction/ptosis (one or both may occur)
- Lower eyelid laxity
- The fullness of the lower eyelid and shallowing of the inferior fornix (caused by the rotation of the orbital tissues that occur in an enucleated socket)
- Posterior tilt of the prosthesis
Enophthalmos may occur early or late. Many methods have been described at restoring the orbital volume. Rose et al. proposed sequential volume replacement, firstly using dermis fat graft or implant followed by a silastic block into the extraperiorbital space.[32] Complications following dermis fat grafts can include graft ulceration, fat atrophy, necrosis, wound dehiscence, granuloma formation, hematoma, and graft surface keratinization. These can be reduced by good surgical technique with careful tissue handling and donor site selection.[33]
Clinical Significance
Enucleation is undertaken in patients with end-stage ocular disease, often with no visual potential and in pain. It can be a life-saving procedure in intraocular malignancy and vision-sparing in cases of sympathetic ophthalmia. However, the decision to proceed to enucleation is often a difficult one to make for the patient and the healthcare team. There are many differences in techniques and implant materials, which can affect the incidence and location of any postoperative orbital and adnexal complications.
Enhancing Healthcare Team Outcomes
Enucleation requires an interprofessional team consisting of a surgeon, ophthalmic nurse, theatre staff, and medical assistants. The team will identify patients suitable for the enucleation procedure and, during the operation, follow safe surgical protocols. They will be able to safely identify and check the correct surgical site prior to undertaking enucleation. The team will also undertake patient education prior to and after the enucleation procedure to ensure good patient rehabilitation and outcomes. [Level 5]
Nursing, Allied Health, and Interprofessional Team Interventions
The interprofessional team will discuss the enucleation procedure with the patient and obtain informed consent prior to surgery. The patient's expectations, concerns, and psychological welfare regarding eye removal will be explored. Instrumentation and correct site checks will be performed by the theatre team. Communication with other team members before and after the procedure contributes to safe care standards. [Level 5]
Nursing, Allied Health, and Interprofessional Team Monitoring
There are early and late postoperative complications associated with the enucleation procedure. The multidisciplinary team will inform and maintain communication with the patient through the postoperative period to ensure they can manage their wound care, and be aware of red-flag symptoms upon which they should present back to the surgical team. The healthcare team will be closely involved in the patient review process in the hospital outpatients. [Level 5]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
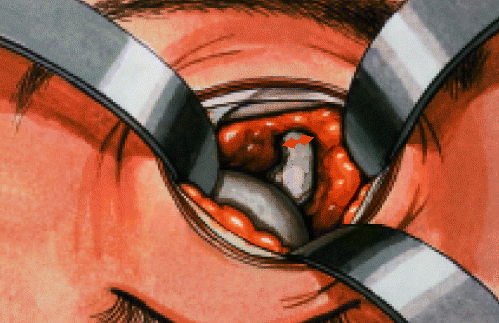
Enucleation: technique of the supero-medial approach to the optic nerve so that the optic nerve may be cut under direct vision, thereby avoiding the risk of transecting the globe in the presence of an introcular tumor. it also allows one to obtain an adequate length of the optic nerve Contributed by Professor Bhupendra C. K. Patel MD, FRCS
(Click Image to Enlarge)
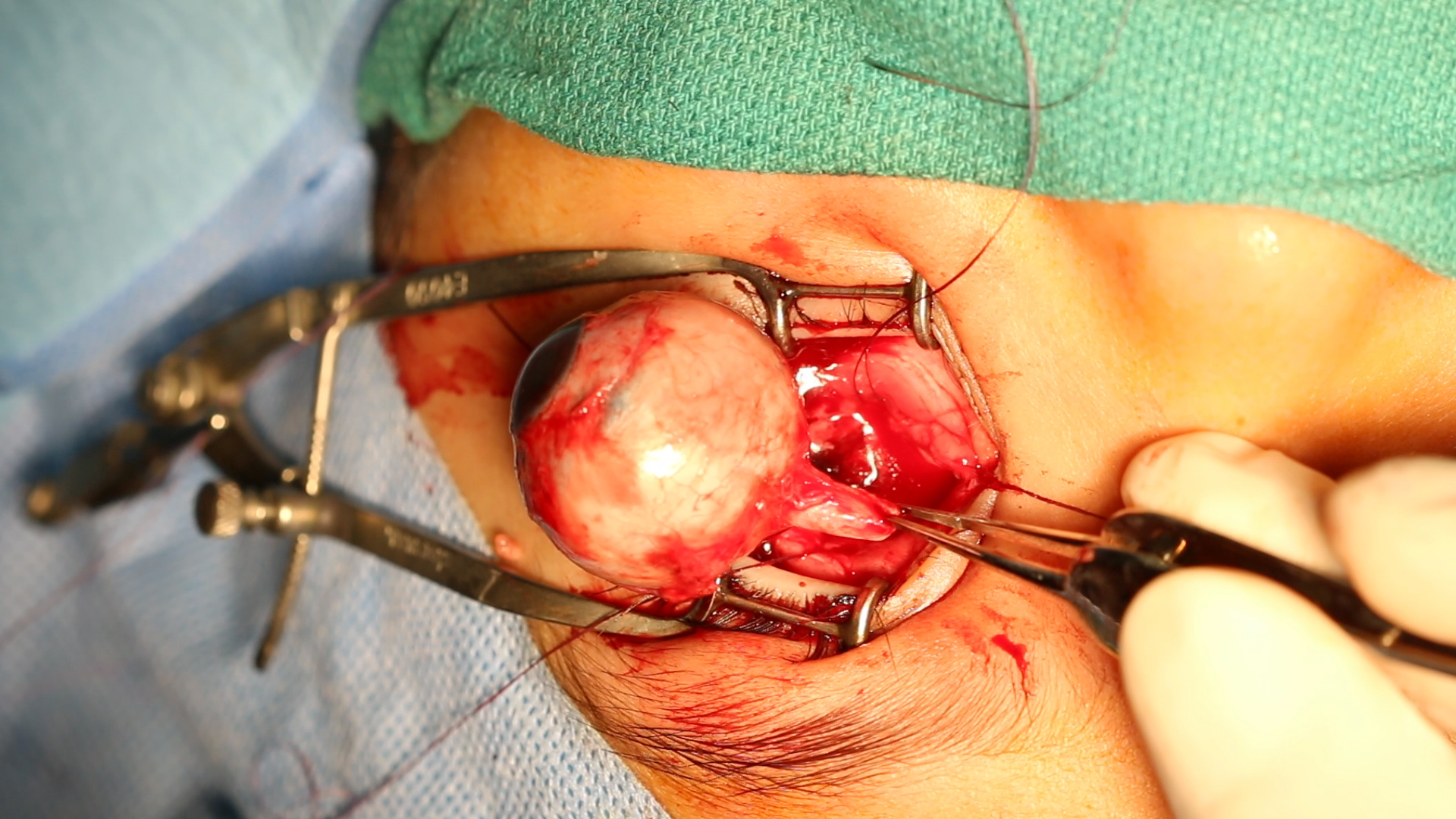
Enucleation. This image shows the enucleation of an eye using the superomedial approach to the optic nerve. This technique preserves sufficient optic nerve length, which is crucial in intraocular malignancies, particularly large retinoblastomas.
Contributed by Professor Bhupendra C. K. Patel MD, FRCS
References
Moshfeghi DM, Moshfeghi AA, Finger PT. Enucleation. Survey of ophthalmology. 2000 Jan-Feb:44(4):277-301 [PubMed PMID: 10667436]
Level 3 (low-level) evidenceJovanovic N, Carniciu AL, Russell WW, Jarocki A, Kahana A. Reconstruction of the Orbit and Anophthalmic Socket Using the Dermis Fat Graft: A Major Review. Ophthalmic plastic and reconstructive surgery. 2020 Nov/Dec:36(6):529-539. doi: 10.1097/IOP.0000000000001610. Epub [PubMed PMID: 32134765]
Borrelli M, Geerling G, Spaniol K, Witt J. Eye Socket Regeneration and Reconstruction. Current eye research. 2020 Mar:45(3):253-264. doi: 10.1080/02713683.2020.1712423. Epub 2020 Jan 10 [PubMed PMID: 31910675]
Tao JP, Aakalu VK, Wladis EJ, Sobel RK, Freitag SK, Foster JA, Yen MT. Bioengineered Acellular Dermal Matrix Spacer Grafts for Lower Eyelid Retraction Repair: A Report by the American Academy of Ophthalmology. Ophthalmology. 2020 May:127(5):689-695. doi: 10.1016/j.ophtha.2019.11.011. Epub 2019 Dec 30 [PubMed PMID: 31899031]
Kashkouli MB, Zolfaghari R, Es'haghi A, Amirsardari A, Abtahi MB, Karimi N, Alemzadeh A, Aghamirsalim M. Tear Film, Lacrimal Drainage System, and Eyelid Findings in Subjects With Anophthalmic Socket Discharge. American journal of ophthalmology. 2016 May:165():33-8. doi: 10.1016/j.ajo.2016.02.016. Epub 2016 Feb 28 [PubMed PMID: 26930225]
Rokohl AC, Trester M, Guo Y, Adler W, Jaeger VK, Loreck N, Mor JM, Pine KR, Heindl LM. Dry anophthalmic socket syndrome - Standardized clinical evaluation of symptoms and signs. The ocular surface. 2020 Jul:18(3):453-459. doi: 10.1016/j.jtos.2020.05.001. Epub 2020 May 6 [PubMed PMID: 32387569]
Custer PL, Trinkaus KM. Volumetric determination of enucleation implant size. American journal of ophthalmology. 1999 Oct:128(4):489-94 [PubMed PMID: 10577591]
Kaltreider SA, Jacobs JL, Hughes MO. Predicting the ideal implant size before enucleation. Ophthalmic plastic and reconstructive surgery. 1999 Jan:15(1):37-43 [PubMed PMID: 9949428]
Level 2 (mid-level) evidenceThaller VT. Enucleation volume measurement. Ophthalmic plastic and reconstructive surgery. 1997 Mar:13(1):18-20 [PubMed PMID: 9076778]
Fountain TR, Goldberger S, Murphree AL. Orbital development after enucleation in early childhood. Ophthalmic plastic and reconstructive surgery. 1999 Jan:15(1):32-6 [PubMed PMID: 9949427]
Kaltreider SA, Peake LR, Carter BT. Pediatric enucleation: analysis of volume replacement. Archives of ophthalmology (Chicago, Ill. : 1960). 2001 Mar:119(3):379-84 [PubMed PMID: 11231771]
Level 2 (mid-level) evidenceDe Potter P, Shields CL, Shields JA, Singh AD. Use of the hydroxyapatite ocular implant in the pediatric population. Archives of ophthalmology (Chicago, Ill. : 1960). 1994 Feb:112(2):208-12 [PubMed PMID: 8311774]
Dharmasena A, Keenan T, Goldacre R, Hall N, Goldacre MJ. Trends over time in the incidence of congenital anophthalmia, microphthalmia and orbital malformation in England: database study. The British journal of ophthalmology. 2017 Jun:101(6):735-739. doi: 10.1136/bjophthalmol-2016-308952. Epub 2016 Sep 6 [PubMed PMID: 27601422]
Shah M, Sun L, Elmann S, Vrcek I, Mancini R, Kim HJ, Carrasco J, Shinder R. Self-inflicted enucleations: Clinical features of seven cases. Orbit (Amsterdam, Netherlands). 2017 Jun:36(3):154-158. doi: 10.1080/01676830.2017.1279670. Epub 2017 Mar 3 [PubMed PMID: 28594303]
Level 3 (low-level) evidenceGündüz AK, Mirzayev I, Temel E, Ünal E, Taçyıldız N, Dinçaslan H, Köse SK, Özalp Ateş FS, Işık MU. A 20-year audit of retinoblastoma treatment outcomes. Eye (London, England). 2020 Oct:34(10):1916-1924. doi: 10.1038/s41433-020-0898-9. Epub 2020 May 6 [PubMed PMID: 32376976]
Maheshwari A, Finger PT. Cancers of the eye. Cancer metastasis reviews. 2018 Dec:37(4):677-690. doi: 10.1007/s10555-018-9762-9. Epub [PubMed PMID: 30203109]
Bergeron E, Lihimdi N, Bergeron D, Landreville S. Orbital recurrence of iris melanoma 21 years after enucleation. BMJ case reports. 2017 Sep 7:2017():. pii: bcr-2017-221137. doi: 10.1136/bcr-2017-221137. Epub 2017 Sep 7 [PubMed PMID: 28882848]
Level 3 (low-level) evidenceTan XL, Seen S, Dutta Majumder P, Ganesh SK, Agarwal M, Soni A, Biswas J, Aggarwal K, Mahendradas P, Gupta V, Ling HS, Teoh S, Pavesio C, Agrawal R. Analysis of 130 Cases of Sympathetic Ophthalmia - A Retrospective Multicenter Case Series. Ocular immunology and inflammation. 2019:27(8):1259-1266. doi: 10.1080/09273948.2018.1517894. Epub 2018 Sep 12 [PubMed PMID: 30207811]
Level 2 (mid-level) evidenceYousuf SJ, Jones LS, Kidwell ED Jr. Enucleation and evisceration: 20 years of experience. Orbit (Amsterdam, Netherlands). 2012 Aug:31(4):211-5. doi: 10.3109/01676830.2011.639477. Epub 2012 May 29 [PubMed PMID: 22642653]
Zheng C, Wu AY. Enucleation versus evisceration in ocular trauma: a retrospective review and study of current literature. Orbit (Amsterdam, Netherlands). 2013 Dec:32(6):356-61. doi: 10.3109/01676830.2013.764452. Epub 2013 Aug 2 [PubMed PMID: 23909276]
Level 2 (mid-level) evidenceAng MJ, Silkiss RZ. The Use of Long-Acting Liposomal Bupivacaine (Exparel) for Postoperative Pain Control Following Enucleation or Evisceration. Ophthalmic plastic and reconstructive surgery. 2018 Nov/Dec:34(6):599. doi: 10.1097/IOP.0000000000001218. Epub [PubMed PMID: 30418397]
Sobti MM, Shams F, Jawaheer L, Cauchi P, Chadha V. Unwrapped hydroxyapatite orbital implants: our experience in 347 cases. Eye (London, England). 2020 Apr:34(4):675-682. doi: 10.1038/s41433-019-0571-3. Epub 2019 Sep 16 [PubMed PMID: 31527766]
Level 3 (low-level) evidenceWladis EJ, Aakalu VK, Sobel RK, Yen MT, Bilyk JR, Mawn LA. Orbital Implants in Enucleation Surgery: A Report by the American Academy of Ophthalmology. Ophthalmology. 2018 Feb:125(2):311-317. doi: 10.1016/j.ophtha.2017.08.006. Epub 2017 Sep 9 [PubMed PMID: 28899574]
Mourits DL, Hartong DT, Bosscha MI, Kloos RJ, Moll AC. Worldwide enucleation techniques and materials for treatment of retinoblastoma: an international survey. PloS one. 2015:10(3):e0121292. doi: 10.1371/journal.pone.0121292. Epub 2015 Mar 13 [PubMed PMID: 25767872]
Level 3 (low-level) evidenceRamey N, Gupta D, Price K, Husain A, Richard M, Woodward J. Comparison of complication rates of porous anophthalmic orbital implants. Ophthalmic surgery, lasers & imaging : the official journal of the International Society for Imaging in the Eye. 2011 Sep-Oct:42(5):434-40. doi: 10.3928/15428877-20110812-03. Epub [PubMed PMID: 21899247]
Level 2 (mid-level) evidenceHintschich C, Raithel E, Craig GT, Bernatzky G, Alzner E, Brook IM, Collin R. Glass-ionomer cement: evaluation as an orbital implant. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 1999 Feb:237(2):169-74 [PubMed PMID: 9987636]
Level 3 (low-level) evidenceRose GE, Collin R. Dermofat grafts to the extraconal orbital space. The British journal of ophthalmology. 1992 Jul:76(7):408-11 [PubMed PMID: 1627511]
Pelton RW, Patel BC. Superomedial lid crease approach to the medial intraconal space: a new technique for access to the optic nerve and central space. Ophthalmic plastic and reconstructive surgery. 2001 Jul:17(4):241-53 [PubMed PMID: 11476174]
Level 3 (low-level) evidenceJordan DR, Brownstein S, Jolly SS. Abscessed hydroxyapatite orbital implants. A report of two cases. Ophthalmology. 1996 Nov:103(11):1784-7 [PubMed PMID: 8942870]
Level 3 (low-level) evidenceSoparkar CN, Patrinely JR. Abscessed hydroxyapatite orbital implants. Ophthalmology. 1997 Jul:104(7):1059 [PubMed PMID: 9224451]
Level 3 (low-level) evidenceSmit TJ, Koornneef L, Zonneveld FW, Groet E, Otto AJ. Computed tomography in the assessment of the postenucleation socket syndrome. Ophthalmology. 1990 Oct:97(10):1347-51 [PubMed PMID: 2243686]
Rose GE, Sigurdsson H, Collin R. The volume-deficient orbit: clinical characteristics, surgical management, and results after extraperiorbital implantation of Silastic block. The British journal of ophthalmology. 1990 Sep:74(9):545-50 [PubMed PMID: 2393645]
Bosniak SL. Dermis-fat orbital implantation and complex socket deformities. Advances in ophthalmic plastic and reconstructive surgery. 1992:9():131-41 [PubMed PMID: 1457056]
Level 3 (low-level) evidence