Introduction
The semilunar sensory ganglion (also known as the trigeminal ganglion or Gasserian ganglion) is a thin, crescent-shaped structure in the Meckel cave within the middle cranial fossa.[1] See Image. Semilunar (Trigeminal) Ganglion. The semilunar ganglion is the great sensory ganglion of the fifth cranial nerve (CN V), the largest and most complex of the 12 cranial nerves. The semilunar ganglion plays a critical role in expressing various neuropeptides and signaling molecules important in gene expression, modulation of sensory information, and peripheral and central sensitization.[2]
The ganglion contains the cell bodies of the sensory roots of the 3 major divisions of the trigeminal nerve (CN V); the ophthalmic nerve (V1 sensory), maxillary nerve (V2 sensory), and mandibular nerve (V3 motor and sensory). Trigeminal neuralgia, when refractory to medical treatment, can be effectively treated by percutaneous interventions at the semilunar ganglion by accessing the foramen ovale through the oral cavity and ablating the ganglion by radiofrequency, balloon compression, or chemical means.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
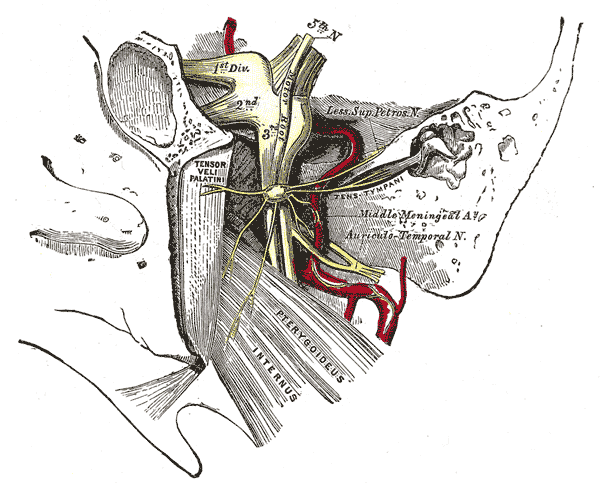
The semilunar ganglion is a crescent-shaped structure located at the anterior, inferior, and lateral borders of the Meckel cave, a dural recess formed between the dura propria and periosteal dura of the middle cranial fossa.[3] It acts as a conduit between the middle and posterior fossae, which connects the prepontine cistern and the cavernous sinus.[4] The Meckel cave is situated within the trigeminal impression at the petrous apex of the temporal bone and is near the carotid canal inferolaterally, and its upper anterior part borders the fibrous lateral wall of the cavernous sinus.[4][5][4] A further important relationship is with the superior petrosal sinus, which passes directly over the trigeminal ganglion within the Meckel cave.[6] Thus, the cave is a dural space containing the semilunar (trigeminal) ganglion.
The convex border of the semilunar ganglion is located at the antero-infero-lateral walls of the Meckel cave, while the concave border, which is also known as the sinus ganglion, is located postero-supero-medially in the direction of the trigeminal cistern and the ostium of the Meckel cave. A layer of dura propria and arachnoid surrounds the semilunar ganglion. The posteromedial portion of the semilunar ganglion is situated in the trigeminal cistern while the anteroinferior portion is located outside of the trigeminal cistern as it adheres closely to the dura of the the Meckel cavity medially, and the dura of the temporal fossa laterally.[1]
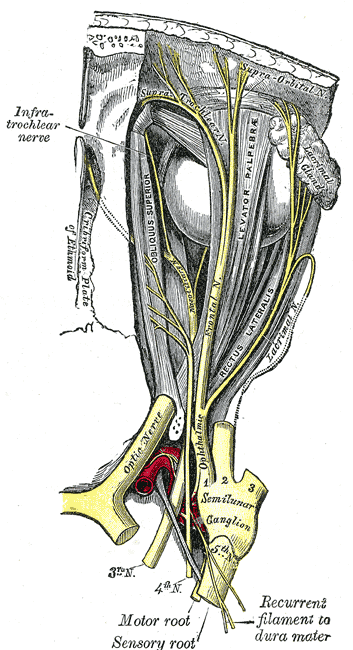
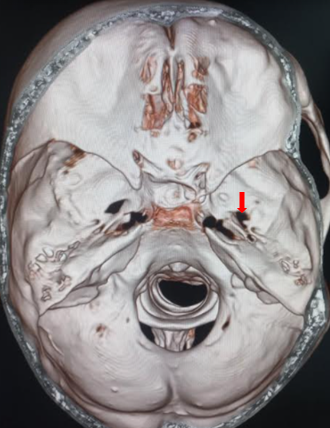
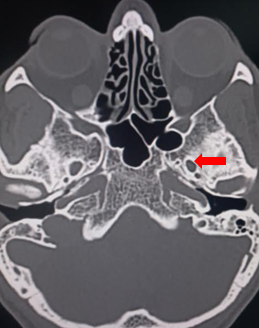
The semilunar ganglion segregates into 3 divisions: the ophthalmic (V1), which is the dorsal division, maxillary (V2), which is the intermediate portion, and the mandibular (V3), which is the ventral division. The ophthalmic portion exits the semilunar ganglion and enters the orbit via the superior orbital fissure. It separates into the supraorbital, supratrochlear, and nasociliary nerves, providing innervation to the nose and forehead. The maxillary portion leaves the middle cranial fossa by passing through the foramen rotundum before branching multiple times to provide innervation via the infraorbital, palatine, zygomatic, and superior alveolar nerves.[7] The mandibular division exits the cranium through the foramen ovale and separates into the buccal, auriculotemporal, lingual, and inferior alveolar nerves (see Image. Foramen Ovale).[8] It is the trigeminal nerve's only division that provides sensory and motor components.
The cellular composition of the semilunar ganglion is between 20,000 to 35,000 pseudo-unipolar neurons, with non-neuronal cells, such as glia and fibroblasts, outnumbering them by approximately 100 times.[9] The semilunar ganglion is essential for releasing many neuropeptides and signaling molecules. As a potent vasodilator, calcitonin gene-related peptide is the most prevalent among these neuropeptides and plays a vital role in the pathophysiology of cluster and migraine headaches.[10] The semilunar ganglion also expresses many signaling molecules, including adenosine triphosphate, neurotrophic factors, nitric oxide, and cytokines, that target nearby neurons or satellite glial cells. These signaling molecules and neurotrophic factors play an important role in peripheral and central sensitization, such as in the case of primary headaches.[2]
Embryology
The semilunar ganglion contains neurons that arise from 2 trigeminal placodes and the neural crest. In the chick, they are known as the ophthalmic and maxillomandibular trigeminal placodes, whereas in the Xenopus frog, they are known as the profundal and trigeminal placodes. In the chick, the ophthalmic placode has a small area of ectodermal thickenings divided across a broad area rostral to rhombomere one and gives rise to postmitotic neurons.[11] The maxillomandibular placode is a much thicker area of ectodermal thickenings that is ventral to rhombomeres 2 and 3 and gives rise to mitotically active neurons.[12]
The first condensation of the semilunar ganglion forms by neural crest cells. In the chick, the ophthalmic placode can express Pax3 (also known as the paired domain transcription factor gene), Fgfr4, (also known as the fibroblast growth factor receptor 4 gene), and Neurogenin2 (also known as the proneural transcription factor gene) while the maxillomandibular trigeminal placode expresses Ngn1 (also known as the proneural transcription factor gene). Neurogenesis in the ophthalmic placode starts in the ten-somite stage with the expression of Ngn2. Researchers also found that even though the degree of separation between the ophthalmic and maxillomandibular trigeminal placodes augments with time, a definitive border does not exist even at the 16-somite level. Moreover, some cells along the border between the 2 placodes express Pax3 and Ngn1, which suggests these cells respond to ophthalmic and maxillomandibular placode-inducing signals.[12]
Blood Supply and Lymphatics
Blood supply to the semilunar ganglion comes from the middle meningeal artery, accessory middle meningeal artery, the inferolateral trunk of the internal carotid artery, and the tentorial branch of the superior cerebellar artery. The trigeminal nerve root acquires vascular supply from the superolateral pontine artery, anterior inferior cerebellar artery, inferolateral or posterolateral pontine arteries, superior cerebellar artery, basilar artery, and trigeminocerebellar artery. The motor root and the ophthalmic portion of the sensory root usually receive blood supply from the superolateral pontine artery while the maxillary portion receives blood supply from the superolateral and inferolateral pontine arteries and the mandibular portion of the anterior inferior cerebellar artery. Most of the blood supply to the semilunar ganglion is supplied by the middle meningeal artery and inferolateral trunk of the internal carotid artery.[13] As discussed, the superior petrosal sinus lies near the Meckel cave and provides venous drainage of the cavernous sinus. It passes posterolaterally from this point to drain into the junction of the sigmoid and transverse sinuses.[6] It runs in the superior petrosal sulcus of the petrous part of the temporal bone, adjacent to the trigeminal impression, in which the semilunar ganglion lies within the Meckel cave.
Muscles
Only the mandibular branch of the trigeminal nerve has a motor component. The mandibular branch provides motor innervation to the facial muscles involved in mastication, including the master, temporalis muscle, and lateral and medial pterygoids.[14] It also separates into branches that innervate the tensor tympani, tensor vela palatine, mylohyoid, and the anterior part of the digastric muscle.[15] Masticatory weakness is a recognized complication following percutaneous balloon compression or ablation of the semilunar ganglion, albeit at a relatively low rate. It has been reported to present in around 1% of patients following percutaneous balloon compression and typically resolves in the follow-up period over some weeks.[16]
Physiologic Variants
Reports indicate that the size of the semilunar ganglion varies from 14 to 22 mm in length and 4 to 5 mm in thickness. However, after considering the concave structure of the semilunar ganglion, the semilunar ganglion is 1.5 to 2 mm in thickness.[1]
Surgical Considerations
Targeting the semilunar ganglion in the Meckel cave by utilizing anatomic landmarks or image-guided by fluoroscopy or computed tomography may be effective for patients with refractory facial pain due to trigeminal neuralgia, certain headaches, conditions such as recalcitrant herpes zoster ophthalmicus and postherpetic neuralgia. This option is generally considered when medical management with maximal oral analgesia (typically carbamazepine) has been trialed and not provided adequate pain relief. The procedure may also be an option when neurosurgical management is impractical.
The procedure was first described by Belber et al in 1987 [17] and has since become widely accepted as safe and efficacious in treatment-resistant trigeminal neuralgia.[18][19] The procedure involves access via the mouth with a needle to enter the foramen ovale and the Meckel cave (see Image. Foramen Ovale, Computed Tomography). The patient is placed in the supine position at 30 degrees, and intraoperative fluoroscopy is used to confirm the successful placement of the cannula.[19] The Hartel anatomical landmarks are used: the entry point through the skin is 2.5cm lateral from the angle of the mouth, with a trajectory towards the medial border of the ipsilateral pupil, intersecting a line approximately 2.5cm anteriorly from the external auditory meatus.[20]
Once access is gained, either balloon compression, glycerol infiltration, or radiofrequency thermocoagulation of the semilunar ganglion can be performed to provide a therapeutic effect.[21][22][23][22][21] Meta-analysis of the different techniques shows that all 3 techniques are largely equal concerning their ability to relieve pain.[24][25] However, some of the post-operative risks are more nuanced. Patients undergoing balloon compression were found to be at higher risk of post-operative mastication weakness and trochlear nerve weakness (see Image. Trochlear Nerve, Nerves of the Orbit).[26] Patients undergoing radiofrequency ablation, however, were found to have increased rates of post-operative trigeminal anesthesia but, conversely, lower rates of herpes reactivation.[26] Although providing a significantly less invasive option for treating trigeminal neuralgia, the 3 percutaneous techniques do not offer significantly reduced complication rates compared to the more invasive operative technique of microvascular decompression.[27][28]
Clinical Significance
As stated previously, the semilunar ganglion plays a critical role in expressing various neuropeptides and signaling molecules important in gene expression, modulation of sensory information, and peripheral and central sensitization.[29] As a potent vasodilator, calcitonin gene-related peptide is the most prevalent among these neuropeptides and plays an essential role in the pathophysiology of cluster headaches.[2][30] The semilunar ganglion also expresses many signaling molecules, including adenosine triphosphate, neurotrophic factors, nitric oxide, and cytokines, that target nearby neurons or satellite glial cells.[10]
The semilunar ganglion is also known to become latently infected with the herpes simplex virus type-1 (HSV-1).[31] Herpes simplex virus type-1 would periodically reactivate, whereas the varicella-zoster virus rarely reactivates itself. The reactivation of this virus generally results in various cranial nerve disorders, including vestibular neuritis, Bell palsy, and herpes labialis. Also, as stated previously, neural block of the semilunar ganglion may be effective for patients with refractory facial pain, certain headaches, and other conditions, such as recalcitrant herpes zoster ophthalmic and postherpetic neuralgia who have not responded well to other means. The semilunar ganglion is also involved in the often underappreciated perineural spread of tumors, which are the most common malignant tumors in which there is delayed diagnosis. This situation is a well-recognized phenomenon in head and neck cancers, including squamous cell carcinoma, adenocystic carcinoma, lymphoma, and rhabdomyosarcoma.[14]
The semilunar ganglion is also known to incur damage in patients with trigeminal trophic syndrome (TTS).[32] Commonly seen in elderly patients, TTS is a form of chronic facial ulceration that usually appears in a unilateral crescent-shaped form near the nasal ala and, in more serious cases, in the jaw, forehead, cheeks, and lips. Patients with TTS may report paraesthesia, including burning or shooting pains. The general belief is that ulceration might result from the patient touching their face consistently, especially during sleep. Some treatment options include altering the patient’s behavior, occlusive dressings, oral psychotropic medications, and arm splints.[32]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Yousry I, Moriggl B, Schmid UD, Naidich TP, Yousry TA. Trigeminal ganglion and its divisions: detailed anatomic MR imaging with contrast-enhanced 3D constructive interference in the steady state sequences. AJNR. American journal of neuroradiology. 2005 May:26(5):1128-35 [PubMed PMID: 15891171]
Messlinger K, Russo AF. Current understanding of trigeminal ganglion structure and function in headache. Cephalalgia : an international journal of headache. 2019 Nov:39(13):1661-1674. doi: 10.1177/0333102418786261. Epub 2018 Jul 10 [PubMed PMID: 29989427]
Level 3 (low-level) evidenceYoussef S, Kim EY, Aziz KM, Hemida S, Keller JT, van Loveren HR. The subtemporal interdural approach to dumbbell-shaped trigeminal schwannomas: cadaveric prosection. Neurosurgery. 2006 Oct:59(4 Suppl 2):ONS270-7; discussion ONS277-8 [PubMed PMID: 17041497]
Malhotra A, Tu L, Kalra VB, Wu X, Mian A, Mangla R, Michaelides E, Sanelli P, Gandhi D. Neuroimaging of Meckel's cave in normal and disease conditions. Insights into imaging. 2018 Aug:9(4):499-510. doi: 10.1007/s13244-018-0604-7. Epub 2018 Apr 18 [PubMed PMID: 29671218]
Sabancı PA, Batay F, Civelek E, Al Mefty O, Husain M, Abdulrauf SI, Karasu A. Meckel's cave. World neurosurgery. 2011 Sep-Oct:76(3-4):335-41; discussion 266-7. doi: 10.1016/j.wneu.2011.03.037. Epub [PubMed PMID: 21986433]
Mortazavi MM, Cox MA, Saker E, Krishnamurthy S, Verma K, Griessenauer CJ, Loukas M, Oskouian RJ, Tubbs RS. The superior petrosal sinus: a review of anatomy, embryology, pathology, and neurosurgical relevance. Neurosurgical review. 2018 Jul:41(3):713-718. doi: 10.1007/s10143-016-0785-9. Epub 2016 Sep 19 [PubMed PMID: 27647276]
Shankland WE 2nd. The trigeminal nerve. Part III: The maxillary division. Cranio : the journal of craniomandibular practice. 2001 Apr:19(2):78-83 [PubMed PMID: 11842868]
Shankland WE 2nd. The trigeminal nerve. Part I: An over-view. Cranio : the journal of craniomandibular practice. 2000 Oct:18(4):238-48 [PubMed PMID: 11202843]
LaGuardia JJ, Cohrs RJ, Gilden DH. Numbers of neurons and non-neuronal cells in human trigeminal ganglia. Neurological research. 2000 Sep:22(6):565-6 [PubMed PMID: 11045016]
Carmine Belin A, Ran C, Edvinsson L. Calcitonin Gene-Related Peptide (CGRP) and Cluster Headache. Brain sciences. 2020 Jan 6:10(1):. doi: 10.3390/brainsci10010030. Epub 2020 Jan 6 [PubMed PMID: 31935868]
Freter S, Fleenor SJ, Freter R, Liu KJ, Begbie J. Cranial neural crest cells form corridors prefiguring sensory neuroblast migration. Development (Cambridge, England). 2013 Sep:140(17):3595-600. doi: 10.1242/dev.091033. Epub [PubMed PMID: 23942515]
Level 3 (low-level) evidenceXu H, Dude CM, Baker CV. Fine-grained fate maps for the ophthalmic and maxillomandibular trigeminal placodes in the chick embryo. Developmental biology. 2008 May 1:317(1):174-86. doi: 10.1016/j.ydbio.2008.02.012. Epub 2008 Feb 21 [PubMed PMID: 18367162]
Level 3 (low-level) evidenceĆetković M, Štimec BV, Mucić D, Dožić A, Ćetković D, Reçi V, Çerkezi S, Ćalasan D, Milisavljević M, Bexheti S. Arterial supply of the trigeminal ganglion, a micromorphological study. Folia morphologica. 2020:79(1):58-64. doi: 10.5603/FM.a2019.0062. Epub 2019 Jul 8 [PubMed PMID: 31282551]
Dankbaar JW, Pameijer FA, Hendrikse J, Schmalfuss IM. Easily detected signs of perineural tumour spread in head and neck cancer. Insights into imaging. 2018 Dec:9(6):1089-1095. doi: 10.1007/s13244-018-0672-8. Epub 2018 Nov 16 [PubMed PMID: 30446949]
Gambeta E, Chichorro JG, Zamponi GW. Trigeminal neuralgia: An overview from pathophysiology to pharmacological treatments. Molecular pain. 2020 Jan-Dec:16():1744806920901890. doi: 10.1177/1744806920901890. Epub [PubMed PMID: 31908187]
Level 3 (low-level) evidenceMontano N, Ioannoni E, Rapisarda A. The risk of mastication weakness after percutaneous balloon compression for the treatment of trigeminal neuralgia. Clinical neurology and neurosurgery. 2020 Aug:195():105880. doi: 10.1016/j.clineuro.2020.105880. Epub 2020 May 4 [PubMed PMID: 32413677]
Belber CJ, Rak RA. Balloon compression rhizolysis in the surgical management of trigeminal neuralgia. Neurosurgery. 1987 Jun:20(6):908-13 [PubMed PMID: 3614571]
Olivero WC, Wang H, Rak R, Sharrock MF. Percutaneous balloon rhizotomy for trigeminal neuralgia using three-dimensional fluoroscopy. World neurosurgery. 2012 Jan:77(1):202.e1-3. doi: 10.1016/j.wneu.2011.03.041. Epub 2011 Nov 18 [PubMed PMID: 22405398]
Level 3 (low-level) evidenceSkirving DJ, Dan NG. A 20-year review of percutaneous balloon compression of the trigeminal ganglion. Journal of neurosurgery. 2001 Jun:94(6):913-7 [PubMed PMID: 11409519]
Level 2 (mid-level) evidenceIwanaga J, Badaloni F, Laws T, Oskouian RJ, Tubbs RS. Anatomic Study of Extracranial Needle Trajectory Using Hartel Technique for Percutaneous Treatment of Trigeminal Neuralgia. World neurosurgery. 2018 Feb:110():e245-e248. doi: 10.1016/j.wneu.2017.10.140. Epub 2017 Nov 22 [PubMed PMID: 29104153]
Baabor MG, Perez-Limonte L. Percutaneous balloon compression of the gasserian ganglion for the treatment of trigeminal neuralgia: personal experience of 206 patients. Acta neurochirurgica. Supplement. 2011:108():251-4. doi: 10.1007/978-3-211-99370-5_39. Epub [PubMed PMID: 21107968]
Level 2 (mid-level) evidenceHarries AM, Mitchell RD. Percutaneous glycerol rhizotomy for trigeminal neuralgia: safety and efficacy of repeat procedures. British journal of neurosurgery. 2011 Apr:25(2):268-72. doi: 10.3109/02688697.2011.558946. Epub [PubMed PMID: 21545328]
Wang Z, Wang Z, Li K, Su X, Du C, Tian Y. Radiofrequency thermocoagulation for the treatment of trigeminal neuralgia. Experimental and therapeutic medicine. 2022 Jan:23(1):17. doi: 10.3892/etm.2021.10939. Epub 2021 Oct 30 [PubMed PMID: 34815769]
Cheng JS, Lim DA, Chang EF, Barbaro NM. A review of percutaneous treatments for trigeminal neuralgia. Neurosurgery. 2014 Mar:10 Suppl 1():25-33; discussion 33. doi: 10.1227/NEU.00000000000001687. Epub [PubMed PMID: 24509496]
Level 3 (low-level) evidenceUdupi BP, Chouhan RS, Dash HH, Bithal PK, Prabhakar H. Comparative evaluation of percutaneous retrogasserian glycerol rhizolysis and radiofrequency thermocoagulation techniques in the management of trigeminal neuralgia. Neurosurgery. 2012 Feb:70(2):407-12; discussion 412-3. doi: 10.1227/NEU.0b013e318233a85f. Epub [PubMed PMID: 21866065]
Level 2 (mid-level) evidenceTexakalidis P, Xenos D, Tora MS, Wetzel JS, Boulis NM. Comparative safety and efficacy of percutaneous approaches for the treatment of trigeminal neuralgia: A systematic review and meta-analysis. Clinical neurology and neurosurgery. 2019 Jul:182():112-122. doi: 10.1016/j.clineuro.2019.05.011. Epub 2019 May 14 [PubMed PMID: 31121470]
Level 2 (mid-level) evidenceNoorani I, Lodge A, Durnford A, Vajramani G, Sparrow O. Comparison of first-time microvascular decompression with percutaneous surgery for trigeminal neuralgia: long-term outcomes and prognostic factors. Acta neurochirurgica. 2021 Jun:163(6):1623-1634. doi: 10.1007/s00701-021-04793-4. Epub 2021 Mar 22 [PubMed PMID: 33751217]
Hannan C, Shoakazemi A, Quigley G. Microvascular Decompression for Trigeminal Neuralgia: A regional unit's experience. The Ulster medical journal. 2018 Jan:87(1):30-33 [PubMed PMID: 29588554]
Yuan H, Spare NM, Silberstein SD. Targeting CGRP for the Prevention of Migraine and Cluster Headache: A Narrative Review. Headache. 2019 Jul:59 Suppl 2():20-32. doi: 10.1111/head.13583. Epub [PubMed PMID: 31291020]
Level 3 (low-level) evidenceGoadsby PJ. Pathophysiology of cluster headache: a trigeminal autonomic cephalgia. The Lancet. Neurology. 2002 Aug:1(4):251-7 [PubMed PMID: 12849458]
Theil D, Derfuss T, Paripovic I, Herberger S, Meinl E, Schueler O, Strupp M, Arbusow V, Brandt T. Latent herpesvirus infection in human trigeminal ganglia causes chronic immune response. The American journal of pathology. 2003 Dec:163(6):2179-84 [PubMed PMID: 14633592]
Fredeking AE, Silverman RA. Successful treatment of trigeminal trophic syndrome in a 6-year-old boy with negative pressure wound therapy. Archives of dermatology. 2008 Aug:144(8):984-6. doi: 10.1001/archderm.144.8.984. Epub [PubMed PMID: 18711069]
Level 3 (low-level) evidence