Introduction
Impression materials are classified as either elastic or non-elastic based on mechanical properties.[1] Elastic materials are capable of stretching, compressing, and recovering after deformation. Elastic materials comprise reversible and irreversible hydrocolloids, addition and condensation silicones, polysulfides, and polyether.[2] Nonelastic or rigid impression materials include impression compounds, zinc oxide eugenol, and impression waxes.[2]
Hydrocolloids were the first elastic materials used in the dental field. Hydrocolloids produce an imprint providing high-definition details despite undercuts. While the impressions deform upon removal, they later adapt to the original shape due to their elastic properties. Alginate is an elastic, irreversible hydrocolloid that offers lower costs, improved patient tolerance, ease of manipulation, reduced execution times, and the possibility of obtaining a detailed impression in a single step.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Composition
Alginate is an irreversible hydrocolloid material that can reproduce soft and hard tissue details when in the presence of water. Alginates are salts produced from the combination of alginic acid with either sodium, calcium, potassium, or magnesium.[3] Alginic acid is a polysaccharide extracted from brown algae, a member of the Phaeophyceae family found primarily in America.[3] Dental alginates are available as a powder designed to be mixed with water. The powder contains sodium or potassium alginates, filler particles, calcium sulfate (reactor), fluoride (accelerator), and sodium phosphate (retarder).[4]
Setting Mechanism
The setting mechanism of alginate is a chemical reaction between the salt sodium alginate and the reactor calcium sulfate.[3] This biphasic chemical reaction has a slowing phase and a setting phase.[3] When the powder is mixed with water, the calcium sulfate reactor initially combines with the sodium phosphate retarder, allowing for adequate working time.[3] After all the retarder is consumed, the remaining calcium sulfate reacts with the alginic salt to form insoluble calcium alginate in gel form.[3]
The retarder can slow the setting reaction, giving rise to dental alginates with different working times. Type I is a fast set that hardens within 1 to 2 minutes, and type II is a standard set that hardens within 2 to 5 minutes.[3] The setting reaction time of dental alginates also depends on the water temperature used for the mixture. Warmer water speeds up the setting reaction, and colder water slows the reaction down.[3] The water-to-powder ratio also affects the setting reaction; the hardening reaction is faster with more powder.[3]
Properties
Alginates are mucostatic impression materials. When freshly spatulated, alginates have low viscosity and record the soft tissues without compressing them. Alginates are easy to manipulate, affordable, and have rapid setting times.[3] Some dental alginates have added features such as the ability to change color to signal the phases of the chemical reaction and different flavors.[3]
However, several downsides of dental alginates have been described. Alginates provide a less accurate reproduction than elastomers; they have low tear strength and are likely to tear when removed from deep undercuts, such as interproximal and subgingival areas; they can only be poured once, producing only one plaster model; and they have poor dimensional stability when pouring is delayed.[3]
An ideal impression material would have sufficient dimensional stability over time to allow for pouring whenever convenient.[5] However, the dimensional stability of alginate impressions decreases with longer storage time.[6] Alginate impressions can undergo imbibition, evaporation, and syneresis, affecting their dimensional stability.[4] Imbibition is fluid absorption by a colloid resulting in swelling, and syneresis is the expulsion of liquid from a gel.[4] Imbibition and evaporation processes decrease when the impression is poured as soon as possible. If it is not feasible to pour the impression immediately, it must be stored in a humid environment. This environment can be created by wrapping the impression in a moistened paper towel before being shipped to the dental laboratory or stored in a container with some water, which will evaporate.[4] Some studies advocate that a damp paper towel should not be placed in the impression if it can be poured within ten minutes to avoid water absorption by the material.[5]
Indications
Alginate is one of the most frequently used materials in dentistry and is the impression material of choice when accurate detail reproduction is not paramount. Alginates can be used to create study casts, preliminary impressions, provisional crown or bridge impressions, opposing arch impressions, and fabricate orthodontic casts, sports mouth guards, occlusal splints, and bleaching trays.[3][7] The use of irreversible hydrocolloids is limited in permanent crown and bridge work due to poor tear strength: compressive strength is adequate but tensile strength is poor.
Contraindications
Very few allergic reactions to alginates have been reported. Alginate impression material is contraindicated in patients with a severe allergy to crystalline silica, calcium sulfate, or potassium titanium fluoride.
Technique or Treatment
Selection of Correct Tray
Trays for alginate impressions must have retention, such as perforations.[3] The tray chosen for dental arch impression must be the correct shape and size. Correct sizing allows for the optimum 4 to 6 mm cross-sectional thickness of the alginate.[5] Alginic adhesives, available as spray or paint, can also be applied to maximize the material retention in the tray.[3]
Mixing
The mixing method can significantly affect the elastic recovery and tensile strength of the impression. Dental alginates are often manually mixed, but proprietary mechanical mixing devices are available. Alginate is supplied as a powder with a measuring scoop and a cylindrical cup for measuring water. The recommended powder-to-water ratio is usually 1:1 but is set by the manufacturer.
Manual mixing is performed by adding the measured powder to a flexible rubber bowl and subsequently adding the corresponding amount of water. Mixing must be rapid, wiping or stropping against the side of the rubber bowl with a wide-blade spatula. The final mixture must have a creamy consistency but should not fall from the spatula when lifted.[7] The mixing time is important. Generally, 45 to 60 seconds is sufficient; total mixing time is dictated by the brand and type of alginate and a standard or fast set. The mixing time for regular alginate is 1 minute, and fast-set alginate should be mixed for no more than 45 seconds. The time must be carefully monitored; undermixing and overmixing affect the strength of the set impression.
Manufacturers have introduced a "dustless" alginate designed to reduce the dust encountered after tumbling; the powder is thicker and less prone to become airborne. In addition, color indicators have been added to some formulations to identify setting reactions, allowing the operator to decide when to proceed to the next step of impression-making.
Semiautomatic mechanical mixing devices, available since 1978, create a less viscous mixture with fewer bubbles.[8] Mechanical mixing may also improve the compressive strength and elastic recovery of alginates.[9]
Loading
Alginic adhesives are required to dry for five minutes and must be applied before preparing the alginate.[3] Rinsing the mouth with a mixture of mouthwash and water before the impression is taken removes mucin and reduces the surface tension, avoiding air bubbles.[7] The freshly mixed alginate is loaded into the previously selected tray. Any excess material should be removed using the spatula. Some remaining fresh material can be placed on the occlusal surfaces of the teeth to be impressed to improve the reproduction of occlusal anatomy. A small amount of alginate can also be put on the palate.
Impression Taking
When placing the tray in the mouth, retract one side of the lip using a mouth mirror or gloved finger.[9] Insert the tray in the mouth from the side opposite retraction.[9] Center the tray handle and hold the tray in place using light pressure until the material sets; excess pressure during setting results in strain.[9] Relieve the labial flange by manipulating the lips.
Remove the set impression with a quick snap, breaking the seal between the oral tissues and the tray by gently pushing with the finger or applying air directly into the buccal sulcus and then pulling with downward pressure. Rinse the impression with cold water to remove any surface saliva, debris, or blood. Disinfect the rinsed tray.
Disinfection of Impressions
Impression materials will contact saliva and blood during impression taking. Oral microorganisms and other infectious pathogens can be transmitted from impressions to dental laboratories.[10][11][12][13] Dentists and dental nurses are responsible for disinfecting the impression before pouring it with gypsum or sending it to the dental technician.
The first step in disinfecting impressions is to rinse them well with tap water, which removes a significant number of microorganisms from the impression.[5] The most effective method for disinfecting an impression is by immersing it in a disinfectant liquid for 30 minutes.[14] This is more advantageous for hydrophobic materials because they only experience a slight distortion when disinfected this way.[14] By contrast, irreversible hydrocolloids are hydrophilic materials and undergo distortion when immersed in a solution.
Alginates are usually disinfected during the preparation step by spraying or mixing the alginate powder with a disinfectant solution instead of water, allowing the impression to be poured immediately.[14] However, mixing alginate with disinfectant solutions may affect its properties, depending on the type and concentration of the disinfectant agent.[10] Studies indicate that chlorhexidine is the most suitable disinfection agent to mix with alginate as it did not significantly alter the properties of the material.[10]
Disinfected gypsum die stones may also be used, avoiding the distortion experienced by the impression material during other disinfection maneuvers.[15]
Clinical Significance
Alginate impressions play a key role in daily dental practice due to their easy manipulation, fast set times, and affordable price. Understanding the properties, mode of setting, and manipulation of dental alginates is essential to achieve correct and predictable impressions, translating into good dental casts and avoiding the need to repeat the impression. Furthermore, decontaminating and disinfecting dental impressions to decrease the risk of cross-contamination must be emphasized. The selection of the appropriate disinfection method is dictated by the properties of the material, namely imbibition, syneresis, and evaporation. Thorough knowledge of the mechanical properties of alginates enables clinicians to selectively utilize these materials to optimize outcomes.
Enhancing Healthcare Team Outcomes
Creating dental impressions and the resulting dental casts is one of the best examples of collaborative work between dental clinicians, nurses, and laboratory technicians. The dentist selects the appropriate material and corresponding stock tray for the clinical case. The dental assistant usually mixes and loads the freshly made alginate into the tray. The dental assistants are also responsible for cleaning and disinfecting the impressions and either pouring them with gypsum or packing them to send to the laboratory. The package must contain documentation of adequate disinfection. The technician fabricates the impression if the impression arrives at the lab unpoured.
Understanding the inherent properties of irreversible hydrocolloids reduces errors and improves patient outcomes.
Media
(Click Image to Enlarge)
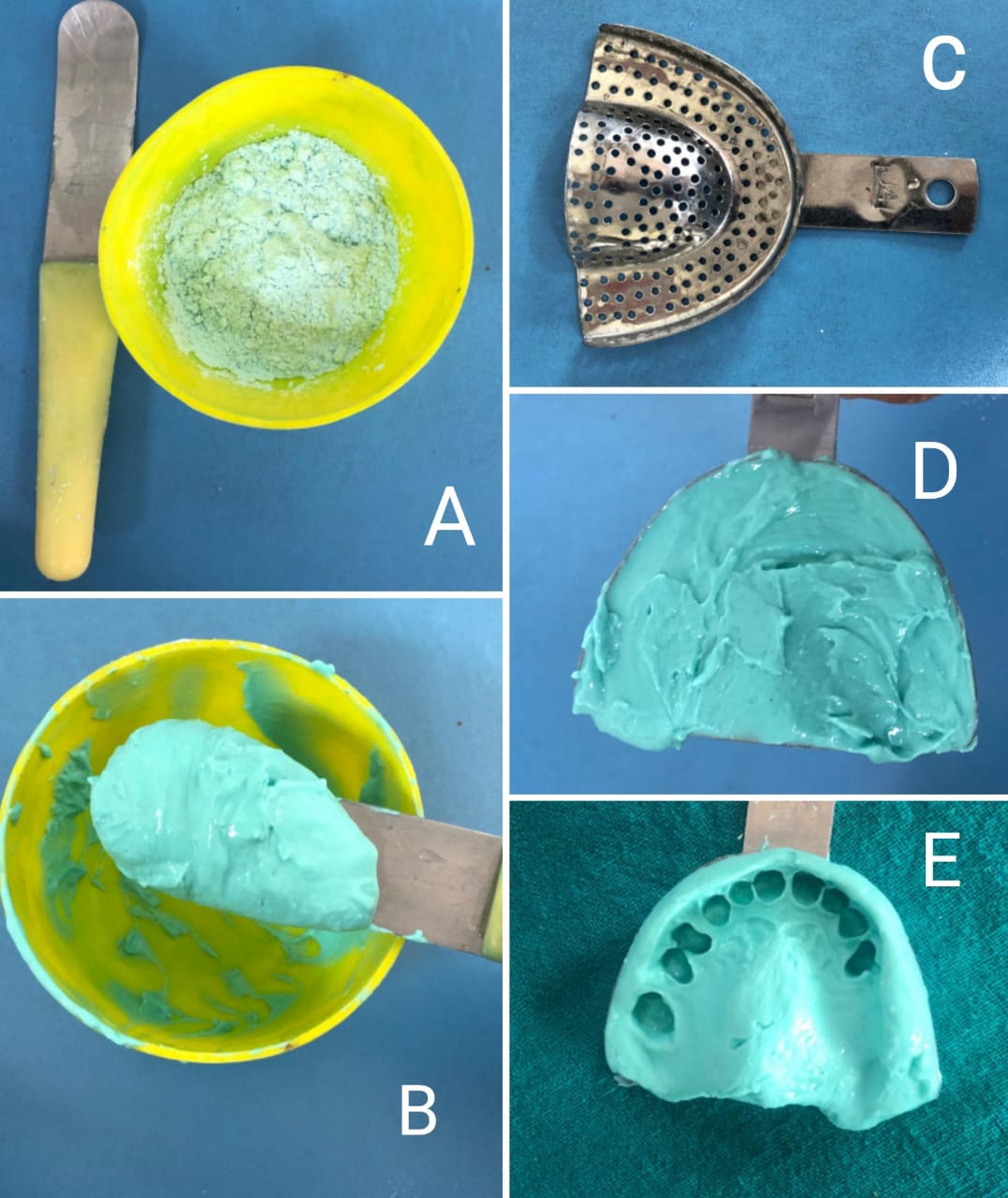
Mixing of alginate for impression making: A) Measured amount of alginate powder in flexible rubber bowl and a curved spatula, B) Mixing is done with appropriate amount of water, C) Perforated impression tray, D) Material loaded into the tray, E) Completed impression Contributed by Dr Ranjan Gupta, MDS
References
Punj A, Bompolaki D, Garaicoa J. Dental Impression Materials and Techniques. Dental clinics of North America. 2017 Oct:61(4):779-796. doi: 10.1016/j.cden.2017.06.004. Epub [PubMed PMID: 28886768]
Gupta R, Brizuela M. Dental Impression Materials. StatPearls. 2024 Jan:(): [PubMed PMID: 34662010]
Cervino G, Fiorillo L, Herford AS, Laino L, Troiano G, Amoroso G, Crimi S, Matarese M, D'Amico C, Nastro Siniscalchi E, Cicciù M. Alginate Materials and Dental Impression Technique: A Current State of the Art and Application to Dental Practice. Marine drugs. 2018 Dec 29:17(1):. doi: 10.3390/md17010018. Epub 2018 Dec 29 [PubMed PMID: 30597945]
Guiraldo RD, Moreti AF, Martinelli J, Berger SB, Meneghel LL, Caixeta RV, Sinhoreti MA. Influence of alginate impression materials and storage time on surface detail reproduction and dimensional accuracy of stone models. Acta odontologica latinoamericana : AOL. 2015:28(2):156-61. doi: 10.1590/S1852-48342015000200010. Epub [PubMed PMID: 26355886]
Donovan TE, Chee WW. A review of contemporary impression materials and techniques. Dental clinics of North America. 2004 Apr:48(2):vi-vii, 445-70 [PubMed PMID: 15172610]
Nassar U, Aziz T, Flores-Mir C. Dimensional stability of irreversible hydrocolloid impression materials as a function of pouring time: a systematic review. The Journal of prosthetic dentistry. 2011 Aug:106(2):126-33. doi: 10.1016/S0022-3913(11)60108-X. Epub [PubMed PMID: 21821167]
Level 1 (high-level) evidenceNandini VV, Venkatesh KV, Nair KC. Alginate impressions: A practical perspective. Journal of conservative dentistry : JCD. 2008 Jan:11(1):37-41. doi: 10.4103/0972-0707.43416. Epub [PubMed PMID: 20142882]
Level 3 (low-level) evidenceInoue K, Song YX, Kamiunten O, Oku J, Terao T, Fujii K. Effect of mixing method on rheological properties of alginate impression materials. Journal of oral rehabilitation. 2002 Jul:29(7):615-9 [PubMed PMID: 12153449]
Frey G, Lu H, Powers J. Effect of mixing methods on mechanical properties of alginate impression materials. Journal of prosthodontics : official journal of the American College of Prosthodontists. 2005 Dec:14(4):221-5 [PubMed PMID: 16359477]
Amalan A, Ginjupalli K, Upadhya N. Evaluation of properties of irreversible hydrocolloid impression materials mixed with disinfectant liquids. Dental research journal. 2013 Jan:10(1):65-73. doi: 10.4103/1735-3327.111795. Epub [PubMed PMID: 23878566]
Owen CP, Goolam R. Disinfection of impression materials to prevent viral cross contamination: a review and a protocol. The International journal of prosthodontics. 1993 Sep-Oct:6(5):480-94 [PubMed PMID: 8297459]
Al Mortadi N, Al-Khatib A, Alzoubi KH, Khabour OF. Disinfection of dental impressions: knowledge and practice among dental technicians. Clinical, cosmetic and investigational dentistry. 2019:11():103-108. doi: 10.2147/CCIDE.S205144. Epub 2019 May 7 [PubMed PMID: 31191035]
Almortadi N, Chadwick RG. Disinfection of dental impressions - compliance to accepted standards. British dental journal. 2010 Dec 18:209(12):607-11. doi: 10.1038/sj.bdj.2010.1134. Epub [PubMed PMID: 21169966]
Poulos JG, Antonoff LR. Disinfection of impressions. Methods and effects on accuracy. The New York state dental journal. 1997 Jun-Jul:63(6):34-6 [PubMed PMID: 9256603]
Donovan T, Chee WW. Preliminary investigation of a disinfected gypsum die stone. The International journal of prosthodontics. 1989 May-Jun:2(3):245-8 [PubMed PMID: 2634410]