Introduction
Vesicoureteral reflux (VUR) is the retrograde urine flow from the urinary bladder to the upper urinary tract, usually during voiding. A short intramural ureter often causes this abnormal backward flow of urine. The clinical significance of VUR was not recognized until 1960, when the condition was associated with recurrent urinary tract infections (UTIs), renal cortical scarring, and permanent kidney damage, particularly in children.[1][2] VUR is the most prevalent urological abnormality in neonates, occurring in approximately 1% of all newborns.[3] However, this percentage rises significantly, up to 15%, in those diagnosed with prenatal hydronephrosis.[4]
VUR is 3 times more prevalent in White than Black patients and twice as likely in women than men, except for cases identified with prenatal hydronephrosis, where VUR is more commonly found in boys. A systematic review of 34 studies indicated that approximately 16% of neonates with ultrasound evidence of hydronephrosis eventually have VUR. When tested, about 30% to 40% of children with a febrile UTI exhibit some degree of VUR, compared to 17% of patients without UTI evidence.[5][6][7][8] In phenotypically male infants with dilated ureters, the odds for the presence of VUR are significantly higher, while in infants with UTI, the prevalence of VUR increases to over 66%.
A genetic predisposition for the disorder exists, as up to two-thirds of children born to women with primary VUR will also exhibit the condition.[9] The incidence of primary VUR is very high in twins.[10] Among siblings, the incidence of VUR is about 30%, although routine screening of asymptomatic siblings with normal renal ultrasound examinations is presently not recommended.[11][12][13] VUR may also be associated with other congenital conditions such as posterior urethral valves, neurogenic bladder, spina bifida, urinary outlet obstruction, bladder overactivity, imperforate anus, ureterocele, and bladder exstrophy.[14][15][16]
VUR can be asymptomatic, unilateral or bilateral, or associated with nephropathy, which can be severe. End-stage renal failure in children due to reflux nephropathy accounts for about 5% of all pediatric renal transplants. However, early diagnosis and timely treatment of VUR can prevent renal damage and recurrent UTIs and salvage the kidneys.[17]
Bladder or bowel dysfunction includes lower urinary tract abnormalities in children, including detrusor overactivity, urinary urgency, urge incontinence, hypoactive bladder, postponement of urination, and other voiding disturbances. These conditions may be accompanied by gastrointestinal and bowel disorders such as encopresis and chronic constipation. Bladder or bowel dysfunction is more commonly found in females.
Patients with VUR with bladder or bowel dysfunction are at a higher risk of developing infections despite continuous prophylaxis and have a much lower rate of spontaneous resolution (31% compared to 61%) and reduced success rates after endoscopic VUR surgical procedures.[18][19][20][21] Treatment for bladder or bowel dysfunction, including timed voiding, laxatives, pelvic floor therapy and exercises, behavioral modifications, and anticholinergic therapy, effectively reduces voiding symptoms, improves bladder function, and increases spontaneous resolution.[22][23]
Approach to Diagnosis
The initial evaluation starts with a urinalysis (dipstick and microscopic), including an evaluation for proteinuria and bacteriuria and a urine culture and sensitivity, if indicated. In addition, obtaining a baseline creatinine level is advisable to establish baseline renal function, particularly in severe cases. Renal ultrasonography is recommended for both initial and follow-up examinations to evaluate renal anatomy, cortical thickness, the presence of hydronephrosis, and any structural abnormalities. Unfortunately, ultrasound lacks high sensitivity or specificity for detecting high-grade VUR.
Technetium-99m–labeled dimercaptosuccinic acid radionuclide renal imaging is recommended to assess kidney function and determine the extent of renal scarring. However, the gold standard for evaluating VUR is direct cystography with voiding cystourethrogram (VCUG).[24][25] VCUG provides detailed anatomical visualization, detects bladder diverticula, identifies ureteral duplications, demonstrates the extent of bladder wall trabeculation, and facilitates VUR grading. Nevertheless, performing VCUG in infants and young children requires specialized expertise to minimize emotional and physical trauma and reduce radiation exposure (see Image. Fluoroscopic Spot Image Revealing Bilateral VUR on VCUG).
The initial imaging method for a child experiencing their first febrile UTI typically involves renal ultrasound, with VCUG being recommended if sonography reveals abnormalities, a second UTI occurs, or if the patient presents with other high-risk factors such as bowel or bladder dysfunction.[26][27] However, neither ultrasound nor radionuclide scanning can replace the VCUG as the definitive imaging modality for VUR.[28] Multiple VCUG imaging cycles (at least 2) are recommended to identify reflux that may only occur early or late in the voiding cycle.[29][30] Notably, it is advised to use diluted contrast at body temperature, administered into the bladder through a small urethral catheter with only gravity pressure. Anesthesia should be avoided as it can impact the results, although limited sedation may be necessary for some patients.
Imaging is performed using the lowest reasonable radiation dose ("as low as reasonably achievable" or "ALARA") to ensure quality imaging, as accurate diagnosis and grading are crucial for selecting optimal therapy. Additionally, urethral spot images during voiding, and scout and post-void films, should be obtained. In addition, it is important to document the maximum contrast in the bladder and the volume at which reflux is initially observed. To ensure optimal VCUG imaging quality with minimal discomfort and risk to the patient, it is advisable to adhere to the 2016 protocol outlined by the American Academy of Pediatrics (AAP) Sections on Urology and Radiology.
The VCUG findings, along with the patient's age and clinical presentation, determine the VUR grading used to formulate the treatment plan. An International Grading System for VUR has been established to streamline clinical decision-making processes.
- Grade I: Reflux solely into the non-dilated ureter.
- Grade II: Reflux into both the ureter and renal pelvis without dilatation.
- Grade III: Reflux with a mildly dilated ureter and pyelocalyceal system with only mild or minimal blunting of the calyces.
- Grade IV: Reflux characterized by a somewhat tortuous, mildly to moderately dilated ureter, with blunting of the renal calyces while preserving the visual impression of the renal papilla.
- Grade V: Reflux involving a very tortuous, severely dilated ureter, and significant pyelocalyceal dilatation, resulting in the loss of renal fornices and no visual papillary impression on imaging.[31][32][33]
Grades III to V are considered dilating or high-grade VUR and are associated with an increased risk of UTIs and renal scarring, especially grades IV and V. Although grade III may be categorized as low, moderate, or intermediate in some grading systems, the consensus opinion typically positions it at the lower end of the high-grade category.
Grading VUR becomes challenging when there is a concurrent urinary tract obstruction, as ureteral dilatation and tortuosity may not solely result from VUR. The presence of obstruction is common and might lead to an overestimation of the VUR grade.[34] Post-void delayed imaging should be obtained if urinary obstruction is suspected.[35] Alternative diagnostic methods include radionuclide cystograms and contrast-enhanced voiding ultrasonography, which can be performed with simultaneous video urodynamics.[36][37][38][39][40][41][42][43][44][45][46] These alternative techniques offer reduced radiation exposure at the expense of anatomical detail. They are particularly valuable for monitoring or tracking VUR, which was previously identified by VCUG. Radionuclide cystograms are especially useful in monitoring patients with high-grade (grade III-V) reflux.[47]
Imaging the upper tracts is crucial, and initial ultrasound imaging can be conveniently conducted in various settings such as clinics, offices, or hospitals. Although a formal VCUG may not always be indicated, ultrasound gives immediate results and is painless, inexpensive, radiation-free, and readily available. Ultrasound imaging of the bladder can detect abnormal changes in bladder wall thickness, detect diverticula, highlight ureteroceles, and measure post-void residual urine volumes. Serial ultrasounds offer an efficient method to monitor renal growth and track changes in ureteral and renal pelvic diameters (RPDs), providing evidence of any hydronephrosis.
Radionuclide studies enable split differential kidney function assessments and offer superior visualization of non-functional (scarred) kidney segments compared to ultrasound. However, intravenous (IV) access is required, and sedation may be necessary for some children. Dimercaptosuccinic acid exhibits higher sensitivity but lower specificity than ultrasound for detecting VUR. In addition, it is recommended to conduct contrast-enhanced ultrasonography and radionuclide scanning before VCUG, allowing patients who test negative on both examinations to potentially avoid a VCUG study.[48][49] Although a dimercaptosuccinic radionuclide scan can effectively rule out high-grade VUR, it often produces false positives. Neither dimercaptosuccinic radionuclide scan nor renal ultrasound alone, or in combination, have demonstrated reliability or accuracy in replacing VCUG for diagnosing VUR.[28]
Baseline ultrasound imaging for siblings of patients with VUR is recommended, as their incidence of VUR is reported to be between 27% and 46%, particularly in those children aged 3 or younger.[13][50][51] Further imaging, including VCUG, should be considered if renal abnormalities are detected or if they experience UTIs.[52] Screening older, toilet-trained siblings without symptoms or UTI history is optional, and children of previously treated patients with VUR are addressed similarly.
The evaluation of prenatal hydronephrosis is primarily based on ultrasonography findings during the third trimester of pregnancy. However, the timing of postnatal follow-up studies varies based on prenatal sonography results and clinical presentation. The severity of hydronephrosis is typically determined by the anterior-posterior RPD observed on a third-trimester ultrasound.[53][54][55][56] For most practical purposes, only those with an RPD of 10 mm or more are considered clinically significant.
- Mild hydronephrosis: RPD <10 mm
- Moderate hydronephrosis: RPD of 10 to 15 mm
- Severe hydronephrosis: RPD >15 mm
Urgent cases that require ultrasonography within the first 48 hours after birth include neonates with significant bilateral hydronephrosis, particularly if accompanied by ureteral dilation or bladder distension, or a markedly hydronephrotic solitary kidney (RPD of 10 mm or more). These instances may demand immediate surgical intervention to safeguard renal function.
In most non-urgent cases, ultrasound is postponed at least 48 hours, as any hydronephrosis is likely to be underestimated due to significant postnatal extracellular fluid changes, which are normal. Mild cases, such as unilateral prenatal hydronephrosis with a normal contralateral kidney, can safely be postponed for 3 to 4 weeks to allow the neonate to become normovolemic. Otherwise, hydronephrosis might be missed due to fluid shifts and dehydration. While some advocate for this approach, others argue that there is little harm in obtaining an earlier ultrasound at 48 hours. This also verifies that the situation does not require more rapid intervention or has significantly deteriorated since the prenatal ultrasound. A follow-up ultrasound examination at 4 to 6 months is recommended in either scenario.
In general, patients with bilateral hydronephrosis and those who demonstrate postnatal hydronephrosis with either ureteral dilation or an anterior-posterior RPD greater than 15 mm should undergo VCUG for further evaluation. Given the significantly higher risk of UTIs in uncircumcised male infants, the decision regarding circumcision for a male child with VUR should be extensively discussed with the parents.[57][58][59][60] Shared decision-making discussions should ensue, ensuring that both the risks and benefits of circumcision are thoroughly reviewed with the family. Families opting against circumcision for their child should receive comprehensive education on proper foreskin care and be thoroughly instructed on recognizing signs and symptoms of UTIs in this age group. Prompt identification and treatment of any new infections are crucial. Long-term follow-up examinations have revealed permanent renal damage in approximately 40% of children initially presenting with moderate or severe hydronephrosis.[61]
Urodynamic and video urodynamic studies may be considered in older children with VUR who are toilet-trained but still have urinary dysfunction or fail prophylactic therapy.[62] These patients may exhibit various forms of neurogenic bladder, such as uninhibited detrusor contractions or detrusor sphincter dyssynergia, which must be addressed and resolved before considering potential surgical intervention.
Approach to Treatment
Managing VUR varies depending on factors such as clinical presentation, patient age, VUR grade, kidney function, UTI frequency, and renal growth patterns. Antibiotic prophylaxis is not indicated in children with a normal urinary tract after a UTI. Accurately predicting which patients will outgrow their VUR without significant permanent renal damage can be challenging.[63] Generally, spontaneous resolution is more likely in patients with lower VUR grades and younger age at diagnosis. Key predictors of additional renal damage and scarring include the severity of reflux, UTI frequency, and the presence of high-pressure bladder conditions. Negative predictive factors for spontaneous resolution of VUR include female gender, higher grade reflux (grades IV and V), other ureteral abnormalities, complete renal duplication, periureteral diverticula, bladder or bowel disorders, and renal pelvic filling without bladder contraction.[64]
Abnormal bowel function significantly increases the risk of UTIs, particularly in patients with VUR and abnormal bladder function.[18][19] About 75% (70% to 80%) of children with grade I and II VUR will spontaneously resolve their VUR by age 5.[65] For grades III and IV, spontaneous resolution is most likely if the reflux is unilateral and discovered before age 2, whereas VUR will resolve in 60% to 70% over 5 years.[66] However, in children over age 5 with bilateral VUR, the rate of spontaneous resolution drops to only 10% to 20% over 5 years. For patients with grade V disease, spontaneous resolution without surgical intervention is rare.[67][68]
The optimal treatment approach for VUR in infants remains a topic of ongoing debate. Many infants with VUR, including those with high-grade reflux, often outgrow the condition spontaneously by age 5. Moreover, up to 30% of boys with VUR experience kidney problems, and infants typically have immature bladder function. Consequently, the focus of treatment has shifted from solely addressing the reflux to preventing UTIs and associated renal damage until the child naturally outgrows the condition. Recently, some experts have advocated for endoscopic surgery as an alternative to conservative approaches like continuous antibiotic prophylaxis. However, endoscopic treatment does not offer significant advantages over antibiotic prophylaxis in preventing new UTIs or renal scarring in most patients. Furthermore, endoscopic intervention carries risks such as anesthesia-related complications, reflux recurrence, bladder perforation, urethral trauma, hematuria, and ureteral obstruction.
Minimally invasive anti-reflux procedures are feasible in infants, but they should not be routinely used due to the high rates of spontaneous resolution of VUR and the immature bladder function in neonates, which reduces the necessity for surgery. Generally, the most effective approach to managing VUR in infants involves closely monitoring them for UTIs and kidney damage. Antibiotic prophylaxis or surgical intervention may be considered if a child experiences recurrent UTIs, shows progressive kidney damage, or exhibits inadequate kidney growth.[69]
In children with high-grade VUR (grades III-V), antibiotic prophylaxis is typically administered to prevent UTIs. In a recent randomized, open-label trial involving over 290 young infants (approximately 75% uncircumcised males with a mean age of 3 months) with high-grade VUR and no prior UTIs, prophylaxis over a 2-year period significantly decreased the proportion of patients experiencing their first symptomatic UTI compared to those receiving a placebo. However, urine cultures from children on prophylaxis showed a higher incidence of antibiotic resistance. Similar findings were observed in the larger Randomized Intervention for Children with Vesicoureteral Reflux (RIVUR) trial. These findings and results from similar studies confirm the benefit of antibiotic prophylaxis in young infants with high-grade VUR and no prior UTIs. However, a closer analysis indicates that patients with grade IV or V reflux, as well as those with combined bladder or bowel dysfunction along with any grade of reflux, received the most benefit from continuous prophylaxis as they have the highest risk.[70][71][72][73][74]
For those patients on prophylaxis, renal imaging (usually ultrasound) is recommended every 6 to 12 months to compare levels of renal scarring, monitor kidney growth, and check cortical thickness. Periodic VCUG or radionuclide cystography should be conducted every 1 to 2 years to assess reflux severity, along with regular blood pressure checks.[75] In asymptomatic children, spontaneous reflux resolution on VCUG indicates cessation of routine imaging.
Continuous antibiotic prophylaxis, while historically the standard of care, is now being reevaluated due to several concerns. These include issues related to adherence patterns and long-term compliance, the necessity for ongoing surveillance, escalating bacterial antibiotic resistance, potential adverse effects on human metabolism, and alterations to the gut microbiome that could contribute to obesity. Additionally, research suggests that prophylaxis may be unnecessary for many children with mild VUR and healthy kidneys.[76][77][78][79][80][81][82][83][84][85]
Certain children with low-grade VUR who are toilet-trained, exhibit normal bladder and bowel function, show no abnormalities on kidney imaging, have been free of UTIs for 1 year, and are asymptomatic.[86][87][88] A proposed predictive computer model has shown promise in identifying approximately 60% of children with VUR who could safely undergo surveillance instead of receiving prophylaxis. However, this model has yet to be validated and is not currently available for clinical use.[89]
The latest guidelines from the American Urological Association (AUA) Pediatric Vesicoureteral Reflux Guidelines Panel recommend continuous antibiotic prophylaxis for children aged 1 or younger with VUR who have a history of febrile UTI, high-grade reflux (grades III-V), or concurrent bladder or bowel dysfunction.[90] Patients at higher risk, such as those with bladder or bowel dysfunction, uncircumcised males, chronic constipation or diarrhea, and grade IV or V reflux, received the most benefit from continuous antibiotic prophylaxis. Compliance with prophylactic treatment is crucial for success, particularly for high-risk patients. Those who strictly adhere to the treatment regimen are 2.5 times less likely to experience a UTI and subsequent renal scarring compared to those with poor compliance.[91]
Continuous prophylaxis was once the standard treatment for VUR but is now considered optional for non-high-risk patients over the age of 1. Current recommendations emphasize individualized therapy following a comprehensive discussion of all treatment options with the family. Patients experiencing recurrent infections or showing signs of progressive kidney damage, scarring, or impaired renal growth should be placed on prophylaxis. They may consider a surgical VUR procedure if prophylaxis fails to safeguard the kidneys and prevent UTIs. In cases where patients on continuous prophylaxis develop a single breakthrough febrile UTI and have healthy, unscarred kidneys, changing the prophylactic agent is a reasonable alternative to surgery.
Recommended prophylactic oral antibiotics: The recommended prophylactic oral antibiotics are listed below.
- Amoxicillin: 12.5 mg/kg daily (recommended for infants aged <3 months)
- Cephalexin: 12.5 mg daily (alternative in neonates aged <2 months or for breakthrough infections as a second agent)
- Nitrofurantoin: 1 mg/kg daily (not for infants aged <1 month or if glomerular filtration rate (GFR) is <40 mL/min)
- Sulfamethoxazole or trimethoprim: 10 mg/kg of sulfamethoxazole and 2 mg/kg of trimethoprim daily (not recommended for infants aged <1 month)
- Trimethoprim: 2 mg/kg daily (not for infants aged <2 months)
Amoxicillin, ampicillin, and cephalosporins are recommended for prophylaxis in neonates <2 months but are not otherwise recommended as primary antimicrobial agents due to their propensity to increase bacterial resistance patterns.[92] They are used in neonates because nitrofurantoin, sulfonamides, and trimethoprim can cause hyperbilirubinemia and other possible adverse effects in these very young children.[93] Prophylactic oral antibiotics are not without problems, such as developing resistant bacterial strains, fungal infections, and antibiotic-related complications, including allergic reactions and pseudomembranous colitis.[94][95] This has prompted investigations into alternative prophylactic therapies that would help avoid oral medications in patients with VUR at risk for UTIs.[96] Continuous antibiotic prophylaxis can generally be discontinued when the VUR spontaneously resolves or after surgical correction.
Intravesical prophylactic antimicrobial instillation has emerged as a viable alternative for managing recurrent bacterial and fungal UTIs in children with VUR. This approach offers several advantages—it avoids systemic complications, minimizes allergic responses, achieves high concentrations of antimicrobials directly in the bladder, allows for the use of nephrotoxic drugs that may not be safe for systemic administration, reduces the development of antibiotic resistance, and preserves the availability of standard antimicrobials for therapeutic intervention in case of infection.
Several studies have demonstrated the effectiveness and safety of intermittent intravesical antimicrobial therapy in both children and adults for UTI prophylaxis.[97][98][99][100][101][102][103][104][105][106][107] Although most pediatric studies are small-scale, larger studies involving adults strongly support the use of intermittent intravesical antimicrobial instillation.[108][109] Gentamicin solutions are the most commonly used, but tobramycin, povidone-iodine, neomycin-polymixin, and neosporin have also been utilized.[110] Notably, systemic absorption of gentamicin has not been observed with intravesical use, and the development of gentamicin-resistant bacteria is exceedingly rare.
Prophylactic intravesical gentamicin solutions: The recommended prophylactic intravesical gentamicin solutions are listed below.
- Gentamicin 8 mg/20 mL or 20 mg/50 mL of 0.9% saline daily for adults and children aged 1 year or older.
- Gentamicin 480 mg/1 L of 0.9% saline should be instilled in 30 to 60 mL daily.[111][112][113]
The use of continuous antibiotic prophylaxis is not without some controversy. The Emilia-Romagna Pediatric Urinary Tract Infections Study Group, comprising a panel of pediatric nephrologists, infectious disease specialists, and pediatric urologists, conducted a comprehensive review of the medical literature spanning 25 years. Based on this extensive analysis, their conclusion is that continuous antibiotic prophylaxis is generally not recommended for most children with VUR, irrespective of grade or infection history, except in cases of significant urinary obstruction where definitive surgery is pending. Their recommendation advocates for vigilant monitoring and prompt treatment of any emerging UTIs in these individuals. This is based on their finding that while the advantages of UTI prevention and reduction of renal scarring were relatively modest, the risk of promoting bacterial resistance was considerably high.[84]
Although the issue remains controversial, in the United States, most practitioners adhere to the AUA Pediatric Vesicoureteral Reflux Guidelines and similar references. These guidelines suggest continuous antibiotic prophylaxis, especially for higher-risk individuals exhibiting bowel or bladder dysfunction, high-grade VUR, progressive renal scarring, recurrent UTIs, or in the case of uncircumcised males.[19][114] To date, no alternative therapy, apart from surgery or continuous prophylactic antibiotics, has demonstrated consistent reliability and effectiveness in UTI prevention among children with VUR. Nonetheless, there is some encouraging evidence regarding the efficacy of cranberry-related products.[90][115][116][117][118][119]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
The ureter is a muscular tubular structure that facilitates the drainage of urine from the kidney to the urinary bladder. In embryonic development, the ureteric bud, also known as the metanephros, originates from the furthest portion of the nephric or Wolffian duct.[120] The ureteric bud eventually makes contact with the metanephric mesenchyme, contributing to kidney formation.[121][122] Proximally, the ureter is continuous with the renal pelvis. As it progresses distally, the ureter enters the wall of the urinary bladder at a slanted angle and forms an oblique tunnel known as the intramural or intravesical ureter. The ureter enters the bladder at the ureteral orifice located slightly lateral and superior to the bladder neck in the trigone. The anatomical connection of the ureter and urinary bladder is called the ureterovesical junction (UVJ).
The normal antireflux mechanism is mostly a passive process and is maintained by the unique anatomy of the UVJ. As the bladder gradually fills, pressure increases on the roof or intravesical side of the intramural ureter, which compresses and closes the lumen, preventing VUR. Any activity that causes an increase in intravesical pressure, such as coughing, sneezing, the Valsalva maneuver, lifting, straining, standing up, or voiding, is immediately compensated by a corresponding increase in intramural ureteral closing pressure, which maintains the antireflux function.[123][124] This mechanism is supported by the contraction of the trigone and the peristaltic action of the ureteral muscles.[125][126]
The approximate optimal ratio of intramural ureter tunnel length to the ureteral diameter to prevent reflux is 5:1, as initially proposed by Paquin.[127] However, recent scrutiny has cast doubt on this ratio, considering the reshaping of the bladder wall and the narrowing and elongation of the intramural ureter as the bladder fills, which may alter this ratio.[128] VUR can manifest as either a primary or secondary pathophysiologic process.[129][130] Primary VUR arises from intrinsic UVJ dysfunction, characterized by abnormally short or incompetent segments of the intravesical ureter. Secondary VUR, on the other hand, develops subsequent to abnormally high voiding pressure in the bladder. Anatomical or functional factors may contribute to secondary VUR.
Primary Vesicoureteral Reflux
Primary VUR is the most common type and is a direct result of the UVJ's failure to function normally. This failure usually takes the anatomical form of a shortened intramural length with an abnormally direct and high lateral connection of the distal ureter to the bladder due to early ureteral budding. Consequently, a short intramural ureter impedes the UVJ's ability to prevent retrograde urinary reflux. While a short intramural ureter is a primary cause of VUR, other factors may contribute. These include ureteral duplication, ectopic ureteral orifices, bladder outlet obstruction, increased intravesical pressure, abnormal trigonal anatomy (diverticulum and ureterocele), and abnormal bladder function. In primary refluxing ureters, ureteral orifices have exhibited atrophy, dysplasia, abnormal smooth muscle fiber architecture, and disruption of the intramural nerve supply.[131]
Although VUR can occur before, during, or after voiding, it is typically observed during micturition. The natural resolution of VUR is believed to stem from the elongation of the intramural ureter as the child matures, alongside tissue remodeling of the UVJ and the maturation of bladder function over time.[123][124]
Intrarenal Reflux (Pyelotubular Backflow)
Intrarenal reflux refers to the backflow of urine into the renal collecting ducts in individuals with VUR.[132][133] This condition poses a significant risk for pyelonephritis, renal scarring, impaired kidney growth, cortical atrophy, childhood hypertension, and chronic renal failure.[134]
Reflux alone does not generally damage the kidney, but when coupled with intrarenal reflux, bacteria from the refluxed urine can infect the renal collecting ducts and parenchyma, leading to potential scarring and infection.[135][136][137] Reflux does not happen uniformly in every calyx and depends on the functional anatomy of the individual papilla and the renal pelvic pressure. Renal damage from UTIs associated with VUR may happen at any age and is not limited to children.[138][139][140][141] Additionally, elevated intravesical pressure associated with VUR might contribute to renal damage.
Simple papillae, characterized by a conical structure, convex surface, and slightly oblique collecting ductal orifices, are less likely to permit intrarenal reflux. Conversely, compound and fused papillae, which often have flat or concave surfaces with widely open collecting duct orifices, are more prone to developing intrarenal reflux. Compound papillae are more commonly found at the extreme upper and lower poles of the kidney, explaining the heightened occurrence of renal scarring in these areas among patients with VUR.[142] Intrarenal reflux in compound papillae can occur with urinary pressure exceeding 35 mm Hg in the renal pelvis.[143][144] Higher pressures may also cause renal scarring without infection, potentially leading simple papillae to develop intrarenal reflux if sustained and sufficiently high renal pelvic pressure persists.[145]
Indications
A VCUG is the preferred imaging modality for diagnosing VUR, and this method is also valuable for postoperative urinary tract evaluation. In children, indications of possible underlying VUR include recurrent UTIs and the first febrile UTI with an abnormal renal ultrasound. Additionally, other indications of VUR in children are prenatal or postnatal urinary tract dilatation (hydronephrosis), dysfunctional voiding, bladder outlet obstruction, neurogenic bladder, dysuria, and hematuria.
Guidelines from the AAP recommend evaluation for VUR in patients aged 2 to 24 months after the second episode of febrile UTI or if their renal ultrasonography is abnormal.[146] Guidelines for evaluating underlying VUR from the National Institute for Health and Care Excellence in the United Kingdom are age-dependent. Accordingly, for infants under 6 months with recurrent or atypical UTIs, further evaluation for VUR is recommended. However, in children aged between 6 months and 3 years, before further evaluation, several criteria should be considered, including a remarkable family history of UTI, a history of a non-Escherichia coli UTI, poor urinary flow, or evidence of hydronephrosis on imaging studies.[147]
In general, continuous antibiotic prophylaxis and surgery offer comparable reductions in the risk of UTIs and renal scarring.[148][149][148] Medical management is usually the initial approach, reserving surgery for cases where medical management fails or is deemed unnecessary, especially in grades I and II VUR. If VUR persists despite medical management, surgical intervention should be considered.
Relative indications for surgical intervention include the following scenarios:
- Failure of adequate renal growth
- Intolerance or noncompliance with antibiotic prophylaxis
- Multiple breakthrough UTIs or pyelonephritis
- Parental request
- Persistent grade IV or V VUR (beyond age 3 has been suggested as a cutoff)
- Progressive renal scarring or kidney failure
Contraindications
Absolute contraindications are not apparent when performing a VCUG. However, relative contraindications to a VCUG include pregnancy and acute UTI. Performing a VCUG during pregnancy exposes the fetus to radiation and is, therefore, not advisable. The VCUG should be delayed until an acute UTI is treated with appropriate antibiotics. Antireflux surgical procedures are generally contraindicated for nonfunctional kidneys, as well as in patients with Hutch diverticula, active voiding dysfunction, and ongoing UTIs. Another contraindication would be a patient who cannot tolerate anesthesia for any reason.
Equipment
A VCUG uses water-soluble, iodinated contrast media and requires a fluoroscopy machine. Recording devices attached to it can capture static or sequenced video images, which are subsequently processed and stored on digital fluorography computers. Radiologists and urologists can then view these images in real time.[31]
Laparoscopic Extravesicular Ureteral Reimplantation for Vesicoureteral Reflux
- 3-0 Synthetic absorbable suture on 26 mm tapered needle
- 3 mm Working ports
- 3 mm Curved insulated rotating scissors
- 3 or 5 mm Laparoscope
- 3 mm Tapered curved jaw dissectors
- 5 mm Hasson trocar
- 5 to 3 mm Reducer seal
- 25 mm Babcock forceps
- 25 mm Working port
- 3 mm Circular Allis grasper
- 3 mm Ratcheted Diamond-flex retractor
- 3 to 5 mm Ratcheted laparoscopic needle driver
Open Extravesical Ureteroneocystostomy
- Scalpels
- Hemostats
- Scissors
- Needle holders
- Tissue forceps
- Retractors
- Surgical drapes and towels
- Sterile gloves and gowns
- Foley catheter
- Suture material
- Ureteral stent
Robotic Ureteroneocystostomy
- Surgical robot with 4 arms: The robotic surgical system includes a console for the surgeon to sit and a robotic cart housing the 4 arms. These arms are equipped with surgical instruments that the surgeon operates from the console.
- Robotic camera: The robotic camera is attached to one of the robotic arms of the robotic surgical system. The camera provides the surgeon with a high-definition view of the surgical site.
- Robotic laparoscopic instruments: These instruments are connected to the other 3 arms of the robotic surgical system and used to perform the actual surgical procedure.
- Additional instruments: Other instruments for this procedure include trocars, Foley catheters, double J stents, suture materials, and surgical clips.
Personnel
The VCUG is performed in the radiology suite under the supervision of a radiologist, with the assistance of a radiology technologist and a nurse. The following personnel are typically involved in the operating room during VUR repair:
- Pediatric urologist: An urologist leads the surgical team and performs the procedure.
- Anesthesiologist: An anesthesiologist or nurse anesthetist administers anesthesia to patients and monitors their vital signs during procedures.
- Surgical technologist or assistant: A surgical technologist assists the surgeon by passing instruments, holding retractors and suction, and providing general surgical technical support.
- Circulating nurse: A circulating nurse manages equipment and supplies in the operating room and assists the surgical technologist.
- Scrub nurse: A scrub nurse assists the surgeon during surgery.
Preparation
Active participation from toilet-trained children and adults is necessary during a VCUG, following the protocol outlined by the AAP Sections on Urology and Radiology. The aim is to capture high-quality images while minimizing patient anxiety and discomfort, particularly as the procedure can be distressing for children. Radiologists or assistants should offer counseling to patients or parents to alleviate fear and anxiety.[150] Distraction and reassurance are often effective non-pharmacological methods to help children relax, although anxious children may require sedation, with midazolam being a commonly used sedative for VCUG.[151]
Patients at risk of developing endocarditis, such as those with prosthetic heart valves or septal defects, should receive antibiotic prophylaxis before undergoing the study. Secondary causes of VUR should be excluded before any intervention. Notably, it is recommended to perform interventions when the bladder size is appropriate, typically that of a 1-year-old patient's bladder. Performing a cystoscopy before the procedure is not necessary or recommended.
Several aspects should be reviewed during the perioperative settings, including, but not limited to, bladder size and function, body habitus, condition and function of the contralateral kidney, patient age, anxiety level, comorbidities, presence or history of nephrolithiasis, relative renal function, severity of UTIs, and whether the patient has a single or duplicated kidney.[26]
Up to 50% of patients with anorectal malformations and imperforate anus have been diagnosed with spinal cord abnormalities, including tethered cord. The severity grade of these lesions correlates with the grading of the rectal lesions. Evidence of VUR and incomplete bladder emptying functions, such as bladder trabeculations (secondary reflux), raises significant concerns for neurogenic bladder dysfunction. Therefore, a comprehensive evaluation, preferably including a spinal magnetic resonance imaging (MRI) scan, is recommended.[152]
Setting Up and Preliminaries
For male patients, it is important to ensure a slight oblique position during urethral imaging to prevent the superimposition of the pelvic bones on the urethra. Frontal projection images should be obtained for women. Lateral imaging should be performed if a urogenital sinus abnormality is suspected. The images should be evaluated for urethral anatomy and function, extravasation, stricture, fistula, and the presence and degree of VUR. Renal fossa imaging should be obtained immediately after voiding, as VUR often occurs during or immediately after voiding.
Cyclical voiding increases the likelihood of detecting VUR. The bladder should be filled with contrast, and the voiding cycle should be repeated 2 to 3 times before removing the catheter. This procedure is routinely performed in children aged 1 or younger due to their lower voiding volumes. VCUG is also indicated for patients with a high probability of having VUR, such as those with recurrent UTIs, Hutch diverticulum, and pyelonephritis. However, a cyclical should not be repeated if VUR is detected during the initial filling.
Cyclical voiding helps in urinary tract dilation. Refluxed contrast is often diluted when mixed with urine in a dilated ureter, which can sometimes be difficult to visualize under fluoroscopy, leading to incorrect grading. Cyclical filling helps to avoid such dilutional effects.[35] A catheter can mask some urethral pathology in men, such as posterior urethral valves and strictures. Therefore, urethral images can be obtained after catheter removal. Before removing the catheter, the bladder should be filled with diluted contrast. After catheter removal, the patient should be instructed to void. However, urethral imaging after catheter removal is unnecessary for female patients.
If the patient is unable to void during the procedure, they may be allowed to use the bathroom if it is located near the examination room. A supine image of the renal fossa should be obtained immediately after voiding, and the time elapsed between the image and voiding should be recorded. Post-void images should be obtained to assess bladder residual volume. In addition, including the renal fossa in the post-void image is important to evaluate reflux immediately after voiding.
Before conducting the study, it is essential to review the patient's clinical history, preliminary studies, and imaging findings. A single supine anteroposterior abdominal radiograph (scout image), including the kidneys, ureters, and bladder, should be obtained. Alternatively, a fluoroscopic spot image can be used. The x-ray is valuable for evaluating any osseous abnormalities associated with VUR, as conditions like spina bifida are often linked with neurogenic bladder and sometimes VUR. Any visible calculi along the urinary tract should be documented.
The Imaging Procedure
A scout image proves helpful to ensure that the abdomen is contrast-free, as any remaining dye from a prior radiological study (eg, a barium enema) may interfere with the VCUG imaging and to evaluate for any artifacts, calcifications, or stones. An experienced nurse or radiology assistant catheterizes the patient with aseptic precautions. The use of anesthetic gel in the urethra minimizes pain and discomfort. The catheter size is usually based on the patient's age, but the smallest usable catheter should be selected. A suggested guide would be 5 French catheters for premature or small infants, 8 French catheters for children aged 1 or older, and 12 French catheters for adolescents or adults.
In patients with a history of urethral strictures or recent urological surgery, a smaller caliber catheter is recommended due to the relatively easier passage through the urethra and bladder with smaller calibers. Notably, it is important to avoid excessive insertion length into the bladder to prevent intravesical looping of the catheter. The catheter should be secured to the perineum or thigh in women and along the dorsum of the penis or pubic symphysis in men, while circumferential taping around the glans should be avoided. After catheterization, the foreskin of the penis should be repositioned to prevent paraphimosis. Finally, ensuring that the bladder is completely drained before commencing the study is crucial.
The water bottle should be connected to the catheter via a tube and positioned at a height that allows for a gravity drip of appropriately diluted contrast. The contrast bottle should be positioned approximately 3 feet above the patient's table. However, the caliber of the tubing may limit the pressure, so raising the height above 3 feet is avoided.
The bottle should be placed at a lower height in patients with recent bladder surgery to maintain lower pressure filling.[31] After confirming the appropriate catheter position under fluoroscopy, contrast is allowed to flow into the bladder. Intermittent pulsed fluoroscopy may be performed to monitor contrast filling into the bladder. Collimation should be used to minimize the radiation dosage to the patient.
In non-toilet-trained patients, the bladder should be filled until voiding occurs. For toilet-trained children and adults, fill the bladder until they feel the physiological urge to micturate. Typically, the bladder is filled to more than the usual estimated capacity. Bladder filling should be stopped if the patient experiences pain or discomfort.
The formula for estimated bladder capacity (in mL) is mentioned below.
- Age 2 or younger: (Weight [in kilograms]) × 7 or (2.5 × age [in months]) + 38
- Age 2 to 14: (Age [in years] × 30) + 30 or (2 + age [in years]) × 30
- Age 14 or older: 500 mL [153]
Fluoroscopic images of the bladder should be obtained in anteroposterior, right anterior oblique, left anterior oblique, and lateral projections both during early filling and when fully distended with contrast. Early filling images are particularly useful for identifying ureteroceles, which may be missed when the bladder is fully distended with contrast. Imaging the full bladder is essential to document its contour and shape, including any wall irregularities, filling defects, or masses within the bladder. In addition, it is noteworthy that a contrast-filled urinary bladder may obscure contrast reflux in the lower ureter when the patient is in the supine position. Lateral imaging helps identify urachal pathology, while oblique projections are useful for demonstrating grade I VUR.
Anteroposterior fluoroscopic spot images should be obtained if reflux occurs. Notably, it is important to include the renal fossa in these images to evaluate the grade of VUR. The volume of the bladder should be recorded at the time of reflux. Oblique images of the bladder should be acquired during reflux to evaluate the insertion of the ureter into the bladder. Patients are instructed to void around the catheter when the bladder reaches its maximum estimated capacity. Adult males typically find it more comfortable to void while standing or with the table tilted to 30° to 35°. Fluoroscopic spot images of the urethra are acquired when the patient begins to void.
Technique or Treatment
Endoscopic Treatment of Vesicoureteral Reflux
In 1984, endoscopic periureteral bulking agents were introduced as a minimally invasive approach to VUR. Patients with grades I to IV of VUR might be suitable candidates for bulking agent management, and successful treatment of some grade V VUR with such agents has been demonstrated.[154]
The endoscopic approach offers numerous benefits, including generally favorable outcomes, minimal invasiveness, limited tissue dissection or harm, low morbidity, outpatient availability, and ease of repeated use if needed. However, the injection technique and bulking agent volume are crucial. Insufficient material may not reduce the reflux, while excessive amounts can lead to ureteral obstruction and hydronephrosis. Younger age, higher reflux grade, and previous unsuccessful endoscopic antireflux procedures are negative predictors of successful reflux resolution via endoscopy.[155]
Bulking agents currently used for endoscopic antireflux surgery include dextranomer-hyaluronic acid gel and polyacrylate-polyalcohol copolymer, which have been used as injectable bulking agents for over a decade, especially for lower-grade VUR, yielding similar outcomes.[156] Although dextranomer-hyaluronic acid copolymer boasts a slightly superior initial success rate, it carries a higher risk of ureteral obstruction and a significant incidence of late reflux recurrence.
The selection of a bulking agent is based on individual surgeon preference and experience. Certain bulking agents have been removed or recalled from the commercial market due to immune reactions, particle migration (such as Teflon), possible malignant potential, and adverse events.
Dextranomere-hyaluronic acid copolymer: Introduced in 2002, the dextranomere-hyaluronic acid copolymer is a viscous gel containing dextranomere microspheres in a hyaluronic acid carrier base. Other features are listed below.
- The microspheres have an intermediate size (80-250 μm in diameter).
- The material is non-biodegradable and appears immunologically inert.
- This material causes minimal scarring and has no apparent malignancy-promoting properties.
- A minimal histological reaction is not apparent to the injected material.
- The copolymer is less likely to cause ureteral obstruction over time.
- The typical injected volume is about 1 mL (ranging from 0.5 to 1.5 mL) and is slightly higher on average than that of polyacrylate polyalcohol.
Overall, the results are somewhat better than polydimethylsiloxane but may not hold up well long-term, with reported high long-term VUR recurrence rates (12%-54%) and minor ureteral obstruction.[157][158]
Polyacrylate polyalcohol copolymer: Introduced in 2010, polyacrylate polyalcohol copolymer is a synthetic, non-biodegradable periureteral bulking agent used for endoscopic periureteral injections to treat VUR.
- The copolymer features a relatively large microsphere size of 320 μm, minimizing particle migration.
- The material's non-biodegradability ensures greater stability and long-term efficacy.
- No apparent malignancy-promoting effect is observed.
- The material tends to form a fibrous capsule with significant scarring.
- Severe inflammation, scarring, and fibrosis typically develop at the injection site, potentially leading to ureteral obstruction long after administration.
- This outcome is attributed to a persistent foreign body reaction, the use of alcohol polymers, and the relatively large microsphere size.
- The treatment may result in ureteral obstruction over time, necessitating long-term follow-up.
- No evidence suggests a malignancy-promoting effect.
- The typical injected volume is about 1 mL (ranging from 0.5-1.5 mL).
- The copolymer may offer better long-term outcomes compared to dextranomer-hyaluronic acid.
- The copolymer may cause ureteral obstruction over time; thus, long-term follow-up is therefore suggested.[159][160]
Techniques of Endoscopic Vesicorureteral Surgery
Three different basic techniques can be used in the endoscopic application of bulking agents—the suburethral transurethral injection (STING) technique, the hydrodistension implantation (HIT) method, and the combined proximal/distal intraluminal injections (double-HIT) procedure.
STING technique: The STING procedure, initially reported in 1981 and later in 1984, is one of the more commonly used endoscopic treatments for VUR. As first described, polytetrafluoroethylene (Teflon) was used as the initial bulking agent, which has now been replaced with newer materials. STING utilizes endoscopic insertion of the injection needle submucosally into the bladder just below (2-3 mm) the ureteral orifice at the 6 o'clock position. As originally described, the needle is advanced within the submucosa for 2 to 3 mm, and the bulking material is injected to create a crescentric ureteral orifice.
Since then, the standard technique has been modified by advancing the tip of the injection needle 4 to 5 mm after insertion, positioning it within the submucosa of the intramural ureter. Injection of the bulking agent elevates the distal intramural ureter, forming a mound that stretches and elongates the UVJ while angulating the ureteral meatus.[161]
Another modification of the STING technique called "ureteral repositioning and injection" begins with the same insertion of the injection needle as the standard STING procedure. However, the needle insertion raises and elevates the distal intraluminal ureter toward the bladder lumen, after which the bulking agent is injected. This variation requires significantly less bulking agent (average of 0.4 versus 0.7 mL), the procedure is simple to perform, and the reported success rate for resolution of VUR is improved compared to the standard STING surgery, up to 91%.[162]
HIT method: The HIT method, introduced by Kirsch in 2004, utilizes hydrostatic pressure to distend the intramural ureteral lumen, facilitating improved visualization. The injection needle is placed within the lumen of the intramural ureter at the 6 o'clock position before traversing the ureter's posterior wall into the submucosal space, where the bulking agent is injected to form a volcano-shaped mound. This technique allows for more optimal placement of the bulking agent due to enhanced needle accuracy, resulting in an augmented antireflux effect within the intramural ureter, extending beyond primarily at the orifice.
The method is often modified to the double HIT technique, where both proximal and distal intraluminal submucosal ureteral injections are performed to improve the antireflux effect further by modifying the entire length of the intramural ureter. The first or more proximal injection is intended to cause coaptation along the UVJ while the bulking compound traverses the ureter, causing partial lumen obstruction. The second, or more distal, injection creates a mound, altering the anatomy and configuration of the ureteral orifice.
More material is required for the injection, but this also increases the reported success rate to an average of 93%.[163] However, additional punctures through the intramural ureteral mucosa may result in leakage of the bulking agent and eventually require an additional endoscopic periureteral bulking procedure. Among the various endoscopic procedures described, the double-HIT is the most commonly utilized method for endoscopic VUR surgery in the United States, possibly being the most effective technique, with an overall resolution of VUR of 82.5% compared to 71.4% with STING.[164][165][166]
A follow-up evaluation, including renal ultrasonography and a VCUG, is recommended 3 months after the procedure. If the original procedure fails, a second endoscopic procedure can be performed regardless of the initial technique. Overall reported success rates with multiple injections in high-volume centers are about 85% but vary from 70% to 100%.[167] Recurrent reflux is not uncommon within 1 year after treatment.
Endoscopic surgical treatment of VUR is considered the preferred initial surgical approach. This approach is minimally invasive, requires minimal anesthesia, and provides an immediate cure for most patients. In addition, it avoids the complications of long-term antibiotic prophylaxis, does not rely on strict patient compliance, has shown durable resolution of grade IV and V reflux, and can be easily repeated if needed.[168][169] Using larger volumes of periureteral bulking agents and multiple injection sites at high-volume centers of excellence increases the success rates of endoscopic treatment.
Predictors of Successful Endoscopic Treatment
Endoscopic periureteral bulking therapy has shown better outcomes under various conditions and circumstances.
- The grade of VUR significantly influences the outcome and is the most crucial factor in predicting successful endoscopic surgery, according to one meta-analysis. Reported success rates in this analysis were as follows:
- Grade I: 89%
- Grade II: 83%
- Grade III: 71%
- Grade IV: 59%
- Grade V: 62%
- A recent study involving over 850 cases of VUR treated endoscopically demonstrated resolution in 70.4% of grade IV cases and 61.9% of grade V cases after a single periureteral injection procedure. Recurrent or residual reflux was resolved in an additional 20.1% after a second injection and 10.4% more after a third injection.
- Another factor to consider is the specific injection technique utilized. Following the initial learning period, the HIT injection method appears to be superior to the STING or modified STING procedures, with success rates of 89% versus 71%. However, some studies have shown no significant difference between the 2 techniques.
- The surgeon's experience with endoscopic injections is an independent predictor of the final success rate. The initial 110 cases show a clear learning curve, with gradual improvement until the success rate stabilizes. Proficiency is typically achieved after at least 20 cases. Despite being generally regarded as a straightforward procedure, technical expertise and experience significantly influence outcomes, especially in complex cases like higher-grade VUR and ureteral duplications.
- Increasing the amount of bulking material typically enhances the final success rate but also leads to higher costs.
- A lower number of previous endoscopic surgical procedures is associated with a higher overall success rate.
- Successful outcomes correlate with proper mound configuration and appearance.
- The presence of bowel or bladder dysfunction negatively predicts VUR control after endoscopic surgery. Untreated abnormal bladder function significantly increases VUR recurrence. Preoperative bladder dysfunction is not a contraindication to endoscopic surgery but should be addressed before surgical treatment.
- Endoscopic treatment is more likely to fail in patients aged younger than 1 and those with existing renal scarring.
- Endoscopic treatment success rates are enhanced by administering larger volumes of periureteral bulking agents and employing multiple injection sites, particularly at high-volume centers of excellence.
- Endoscopic periureteral bulking procedures have been employed in some cases to salvage failed open reimplantation surgery, with a success rate of up to 65%.[64][170][171][172][173][174][175][176][177][178][179][180][181][182][183][184][185]
A Brief History of the Surgical Treatment for Vesicoureteral Reflux
Antireflux surgery was first performed by Hutch on a paraplegic adult patient in 1952.[186] In 1960, Hodson and Edwards first recognized the correlation between VUR in children and frequent UTIs.[2] The intravesical Leadbetter-Politano ureteral reimplantation technique, first introduced in 1958, prioritized creating a new antirefluxing UVJ over reducing bladder neck resistance.[187] This technique eliminated the need for postoperative VCUG surveillance but occasionally led to ureteral kinking. The Glenn-Anderson technique, introduced in 1967, aimed to mitigate this issue.[188]
The modified Leadbetter-Politano technique utilizes extravesical mobilization and transection of the ureter at the level of the UVJ, followed by intravesical reimplantation.[189] Later, in 1975, the Cohen technique introduced cross-trigonal intravesical ureteral reimplantation to address symptomatic VUR.[190] Although this technique is highly successful in relieving VUR, it poses challenges for endoscopic access to the ureters, complicating management when patients develop urolithiasis. Despite this limitation, the procedure remains popular among pediatric urologists due to its success rate, although the inaccessible neo-orifice renders future calculi untreatable via ureteroscopy.
Open intravesical ureteral reimplantations have a success rate of greater than 95%, but these are significant surgeries.[191] Extravesical ureteral reimplantation was established in the early 1960s to mitigate the risk of postoperative hematuria and reduce the likelihood of post-procedural obstruction. In general, ureteral stenting, perivesical drains, and urinary catheters are unnecessary, though transient postoperative retention is possible. Therefore, suprapubic catheter placement is recommended if this procedure is performed on a child who is not yet toilet-trained. Extravesical ureteral reimplantation may be performed via a minimally invasive inguinal approach using a smaller 2.5 cm incision.
Since 2004, robotically assisted minimally invasive ureteral reimplantation has been utilized to manage cases of VUR, but this is technically demanding and usually limited to centers of excellence where sufficient experience is needed.[192] Despite its demanding nature, the procedure boasts a high success rate. Moreover, endoscopic VUR procedures are increasingly favored over traditional open surgical ureteral reimplantation in many centers, except for severe cases.[64]
Standard Non-Endoscopic Ureteral Reimplantation Antireflux Surgeries
Leadbetter-Politano intravesical ureter reimplantation: Although this procedure is technically more complex than other reimplantation methods, it recreates the new ureteric orifice in the usual anatomical position, allowing ureteroscopic procedures in adulthood.[187][193] The peritoneal cavity is accessed in the usual fashion via a Pfannenstiel incision. A longitudinal incision is made in the bladder dome, followed by bilateral fixation of the bladder wall at the rectus fascia. Subsequently, a ureteric stent is placed and secured. A circumferential incision of the ureteric orifice is then performed, along with distal ureterolysis. Mobilization of the adherent peritoneum from the distal ureter ensues, aided by the potential use of a Langenbeck or similar retractor for enhanced visualization.
To create a neo-hiatus, an Overholt or similar forceps is placed close to the bladder wall inside the bladder and 3 cm proximal to the old ureteral orifice over the bladder mucosa. To prepare an adequate neo-hiatus, a free suture is held to provide a guide rail for the ureter. Following the stent removal, one suture end is retracted intravesically, and the other suture is fixed. The completely mobilized ureter is passed extravesically via the neo-hiatus. The distal ureteral segment is transposed through the bladder, and a submucosal tunnel is created, extending from the old to the neo-hiatus. Sutures are used to close the mucosal defect at the old hiatus, and the distal ureteral segment is transferred submucosally. The ureteral orifice is sutured into position, and the bladder mucosa is closed over the ureter. Finally, ureteric stents and cystotomies are placed.[194]
Glenn-Anderson intravesical ureteral reimplantation: The Glenn-Anderson approach begins similarly and is designed to be easier to perform and less likely to cause kinking at the UVJ than the Leadbetter-Politano procedure. After opening the bladder, the ureter is separated from the native attachments and the intramural tunnel. The tunnel is extended or advanced submucosally toward the bladder neck, where the distal end of the ureter is sutured to create a new ureteral orifice. The tunnel is then closed over the ureter.[2][188][195]
Unlike the Leadbetter-Politano technique, the ureter is not removed from the bladder, and a new initial insertion point is not created. The problem with this approach is that the increased length of the submucosal tunnel is relatively modest, but endoscopic access to the ureter is maintained, unlike the Cohen technique described below. In addition, an endoscopic Glenn-Anderson ureteral reimplantation technique has also been described.[196]
Lich-Gregoir extravesical ureteral antireflux procedure: The Lich-Gregoir procedure, introduced in 1964, offers an extravesical method for antireflux surgery. Unlike other techniques, it avoids bladder opening, preserving continuity and vascular supply between the bladder and the refluxing ureter. Moreover, it retains the ureteral orifice in its original position. A modified version of this approach is commonly employed in renal transplantations.[197][198][199][200][201]
A Pfannenstiel or lower midline incision is typically used. The bladder is partially filled and retracted medially to help visualize the ureter. When identified, the ureter is isolated with a vessel loop. Next, the peritoneal reflection is dissected from the external posterior surface of the bladder. An incision is made over the intramural ureter, traversing the serosa and detrusor layers. The incision angle is superior (cephalad) and lateral to the UVJ, covering a distance of 5 cm. Special care is taken to avoid ureteral damage while freeing it from the surrounding detrusor, ensuring the preservation of the ureteral orifice and continuity between the bladder and distal ureter to optimize vascular flow.
Following the incision, a submucosal tunnel is meticulously created between the detrusor and bladder mucosa, extending the length of the ureteral tunnel within the bladder wall. The ureter is carefully positioned within this trough, and absorbable interrupted sutures are used to close the overlying detrusor and serosa. Typically, a Penrose drain is left in place postoperatively. Additionally, there have been descriptions of a transumbilical laparoendoscopic single-site Lich-Gregoir surgery.
Cohen cross-trigonal intravesical ureteral reimplantation: The procedure begins with the conventional entry into the peritoneal cavity via a Pfannenstiel incision, followed by opening the bladder dome longitudinally. A Denis-Browne or similar self-retaining ring retractor is then inserted. Subsequently, a 2-0 or 3-0 absorbable suture is passed through the original ureteral orifice at the 6 o'clock position. Ureteral stents equipped with a 5- or 8-French feeding tube are readied. The periureteral mucosa is incised circumferentially, allowing for complete mobilization of the ureter along its trajectory through the bladder wall. Complete mobilization is advised to ensure up to 8 cm of usable ureteral length.
The muscular ureteral hiatus is dissected on each side, and the former hiatus is closed with absorbable chromic sutures. Bilateral 4 cm mucosal tunnels are prepared. The vascularity of the distal ureteral segments is assessed, with equivocal segments excised if necessary. Subsequently, the ureters are spatulated cephalad and anchored to the intravesical mucosa, followed by closure with 5-0 chromic sutures. In complicated cases of bilateral VUR, ureteral stenting is recommended. Comparative studies have analyzed the Cohen cross-trigonal procedure against the Lich-Gregoir antireflux procedure. While overall success and complication rates are similar, the Lich-Gregoir technique required fewer inpatient hospital days and less operating room time, while also eliminating the need for catheters or ureteral stents.[190][202]
Vesicoscopic Cohen-type cross-trigonal ureteral reimplantation: This surgical technique provides a minimally invasive alternative for the repair of VUR.[203][204] Vesicoscopic refers to a transvesical laparoscopic approach that is performed completely inside the bladder. The patient is positioned in a modified lithotomy position with both thighs elevated and abducted. Cystoscopy is performed in the usual fashion with a 70° angled lens. Three small skin incisions are made superior to the symphysis pubis to facilitate the placement of a midline 6-mm optical trocar and 2 3.9 mm trocars. A large 2-0 transfixion suture is placed. The trocars are inserted intravesically while maintaining traction on the transfixion suture. Subsequently, the cystoscope is withdrawn, and a 30° 5-mm cystoscope is introduced.
The bladder is evacuated of saline and inflated with gas to a pressure of no greater than 7 mm Hg. Bilateral ureteral stenting is performed. Ureters are dissected circumferentially to mobilize a segment of no less than 4 cm or enough length to reach the contralateral ostium. In circumstances of bilateral VUR, submucosal tunneling should be prepared. To reduce tension on the created neo-ureteral orifice, the mobilized ureter is sutured to the bladder entrance. Mucosal defects are closed upon completion of the procedure.
A modified approach to the distal detrusor dissection and ureteral mobilization, involving a restricted periureteral dissection, has been devised to address postoperative urinary retention following bilateral extravesical ureteral reimplantation. Comparative studies between the vesicoscopic Cohen-type cross-trigonal reimplantation and the transumbilical laparoendoscopic single-site Lich-Gregoir procedure indicate similar results, with the Cohen-type procedure deemed technically simpler to execute.[205]
Robotic or laparoscopic extravesicular ureteral reimplantation: Routine cystoscopy and retrograde pyelography are performed to evaluate the location of the ureteral orifices, visualize the length of the posterior bladder wall, and determine the presence of diverticula or ureteroceles. Following these diagnostic preparations, the patient is positioned in a supine decubitus Trendelenburg position. The length of the posterior bladder wall determines the available plane for extravesical detrusor tunneling. Intravesical ureteral reimplantation is preferred when the posterior wall is limited, while patients with ureteroceles are not suitable candidates for extravesical ureteral reimplantation
During the procedure, ureteral stenting enhances the identification of the distal pelvic ureter and is preferred, especially during the procedural learning curve. Additionally, an indwelling Foley catheter is inserted to aid in hydrodistention and facilitate urine drainage. Following this, attention shifts to the abdomen, where pneumoperitoneum is established, and a 5-mm trocar is inserted in the usual fashion.
A 0° laparoscope is preferred due to the minimal interference with the instruments. The pelvis is surveyed, and the anatomy is noted. Three working ports are placed over the imaginary Pfannestiel incisional line, including a 5-mm central port and 2 3-mm lateral ports in the right and left midclavicular lines.[206][207][208] Every precaution must be used to avoid injuries to the inferior epigastric vessels and the dome of the bladder. Subsequently, 4 5-mm ports should be inserted. The umbilical port has 2 ports positioned on each side. Before ureteral dissection, thorough adhesiolysis is necessary to ensure complete exposure. The ureter should be completely exposed from its crossing of the iliac bifurcation to its opening into the bladder. Excessive stripping of the ureter should be avoided to minimize disruption of its vascular supply. The space of Retzius (extraperitoneal retropubic space) should be completely cleared to create a sufficient working space with adequate bladder mobility.
The final reimplantation insertion is placed midway through the course between the broad ligament and the bladder base. The distal ureteral segment is held with 5-mm Babcock forceps to minimize compressive injury. In a male patient, the peritoneum is incised caudal to the vas deferens. The prepared periureteral tissue provides a good working place for a circular-tipped 3-mm Diamond-Flex retractor that assists in bloodless, atraumatic ureteral handling. Otherwise, the dissected ureter can be handled with umbilical tape tied loosely. The entire ureter should be freed from the UVJ while preserving the periureteral tissue. A 5-mm Babcock is recommended for grasping the ureter to avoid unnecessary ureteral trauma from compression effects.
The location of the detrusor muscle tunnel should be outlined with superficial electrocautery markings while the bladder is fully distended, starting from the UVJ and extending along the planned course of the ureter. The ureteral diameter to the tunnel length ratio of 5 or more to 1 should be followed. Clinicians should be aware that a tunnel length of 3 cm is generally sufficient and will probably work adequately in most patients. A 4-0 Prolene suture on a straight cutting needle is placed intraperitoneally as an anchoring suture, with the external end positioned laterally to the midline port to provide appropriate tension for working on the detrusor tunnel.[209]
A detrusor myotomy should be carefully made over the marked line to avoid incising the bladder mucosa. Any mucosal injuries that occur should be immediately repaired. Confirmation of an efficient detrusor trough is achieved by observing mucosal bulging, which prevents ureteral constriction, followed by detrusor flap closure. The mucosal bulge tends to be smaller than in open extravesical ureteroneocystostomy procedures during laparoscopic procedures due to the instillation of a pneumoperitoneum. Working on an overly distended bladder is discouraged as it would limit the working space.
Caudal dissection continues next to the UVJ in an "inverted-Y" configuration to maintain the trigonal attachments and the detrusor tunnel. The ureter is then positioned within the new submucosal tunnel, and the detrusor is reconstructed externally over the ureter using interrupted absorbable sutures.[210] The detrusor repair should be initiated next to the end of the "inverted Y" but should not extend over it to prevent kinking at the ureteral orifice. The final interrupted suture for detrusor reconstruction should be positioned at a distance from the ureter to mitigate the risk of kinking.
Outcomes and Discussion
Endoscopic periureteral bulking procedures for VUR are often preferred initially for patients who fail conservative treatment and require a surgical procedure. They are minimally invasive, allowing for quick recovery, and generally yield good results, particularly with lower-grade VUR. These procedures can be easily repeated if necessary. On the other hand, robotic or open surgical reimplantation procedures offer superior outcomes in high-grade VUR but entail more significant surgeries. Typical reported positive results range from 98% to 99% or higher, except for grade V, where the success rate is around 80%, compared to endoscopic surgery, which typically has success rates roughly 10% to 20% lower.[66][171]
Robotic and open extravesical surgery offer several advantages. The bladder lumen is not opened, which eliminates hematuria, avoids urinary extravasation from bladder leakage, and reduces spasms.[211][212] This accelerates recovery, reduces patient discomfort, and shortens hospital inpatient stays. Moreover, there is less risk of postoperative ureteral obstruction, as there is no need for ureteral stents, suprapubic tubes, or perivesical drains in the absence of ureterovesical anastomosis.
Other advantages of robotic surgery include reduced morbidity, decreased postoperative pain, reduced need for analgesia, quicker recovery after surgery, fewer inpatient hospital days, and minimized abdominal scarring compared to traditional open surgical antireflux procedures. However, robotic surgery is also linked with higher costs, lower overall success rates, and additional complications.[213][214][215][216][217][218][219] A few patients may demonstrate persistent VUR on a postoperative VCUG after a definitive surgical repair. When present, this typically resolves spontaneously without additional treatment.[220]
Successful extravesical and robotic VUR surgery is based on the following principles:
- Complete distal ureteral mobilization should be performed, starting from the peritoneal reflection, while preserving a generous amount of periureteral tissue to maintain the blood supply.
- A long-duration absorbable suture should be utilized to securely anchor the distal ureter in place.
- The detrusor should be opened widely to provide adequate compression when closed firmly over the ureter.
- The intramural tunnel length should be adequate.
In various studies, overall success rates with the newer vesicoscopic ureteral reimplantation procedures are reported to exceed 95%.[196][203][221][222] Follow-up after robotic or open reimplantation surgery typically involves bladder and renal ultrasonography at 3, 12, and 24 months postoperatively. Due to the high success rate of these procedures, a VCUG is generally unnecessary for asymptomatic patients, except perhaps for those with preoperative grade V reflux. However, a VCUG is recommended if the patient experiences a significant febrile UTI or multiple afebrile urinary infections postoperatively.
Complications
Voiding Cystourethrogram
Complications of VCUG include allergic reactions to the contrast media and radiation exposure. If a prior allergic episode to iodinated contrast exists, allergy prophylaxis is recommended, and other imaging studies should be considered. Such allergic reactions are rare if the iodinated contrast remains in the urinary tract and is not absorbed or injected.
Digital imaging and other techniques reduce radiation exposure.[223][224][225] The average fluoroscopy dose is around 0.2 milliSieverts (mSv). The results from a study showed that the average dose from fluoroscopy during a standing VCUG in women approximated 1.1 mSv.[226] Notably, it is important to follow the AAP Sections on Urology and Radiology Protocol guidelines to minimize radiation exposure to the patient. Risks associated with catheter insertion include pain, hematuria, urethral scarring, stricture formation, and UTIs.[26][227]
Complications of continuous antibiotic prophylaxis include hyperbilirubinemia in neonates, increased antibiotic resistance, gastrointestinal disorders, bone marrow depression, and various allergic responses, which may include Stevens-Johnson syndrome.[85][228][229]
Complications of Surgical Procedures for Vesicoureteric Reflux
Complications following endoscopic VUR procedures are rare. Reported issues include ureteral obstruction, dysuria, hematuria, and urinary frequency, which are usually self-limited and resolve spontaneously. Failure to resolve the VUR may be due to inadequate bulking material or migration of the injected agent. In such cases, repeat endoscopic therapy or alternative procedures (robotic or open) may be considered to address significant or persistent reflux postoperatively.
Post-procedural UTIs, persistent reflux, and VUR recurrence have been reported following endoscopic VUR surgical corrective procedures.[230] However, robotic, laparoscopic, and open reimplant procedures for VUR typically have low complication rates. In rare cases, pneumoperitoneum without specific consequences has been observed following minimally invasive vesicoscopic cross-trigonal ureteral reimplantation. In extremely rare circumstances, failure to establish the pneumovesicum has led to early conversion to open laparotomy.[203]
Ureteral obstruction leading to acute hydronephrosis necessitating drainage post-procedure was reported in 0.02% of all patients. Ureteral obstruction and hematuria are recognized postoperative complications of intravesical ureteral reimplantation. Obstruction may arise from factors such as kinking of the distal ureter, angulation at the bladder insertion site, improper placement of the new ureteral meatus, twisting of the ureter, scarring, ureteral ischemia, or an overly constrictive intramural tunnel.
Initially, a nephrostomy tube may manage severe ureteral obstruction, thereby avoiding the need to find and cannulate an edematous ureteral orifice endoscopically. Subsequent antegrade studies can be conducted, and if necessary, the nephrostomy can be replaced with an internal double J stent. If the obstruction fails to resolve spontaneously, formal reimplantation may be required. The most common complication of unilateral repair is the postoperative development of reflux in the contralateral kidney in up to 22% of patients. However, newly developed VUR in the contralateral renal unit is typically mild and resolves independently without surgical intervention.[231]
Additional reported complications include persistent reflux and diverticulum formation. Dysuria, hematuria, failure to correct VUR, migration of bulking agents, and ureteral obstruction leading to hydronephrosis have been reported following endoscopic bulking agent injections and should not be underestimated. Bilateral extravesical ureteral reimplantation carries up to a 4% temporary risk of urinary retention, which may necessitate prolonged catheterization or the use of a temporary suprapubic tube.[167]
Clinical Significance
Untreated VUR often leads to kidney injury, which can manifest as renal scarring, hypertension, or chronic renal disease, and may progress to end-stage kidney failure.[76] Approximately 30% to 40% of infants diagnosed with UTI have underlying VUR.[232] Primary low-grade VUR often resolves spontaneously as children grow and develop.[1] This resolution is attributed to the natural elongation of the intravesical ureter and the strengthening of the intrinsic antireflux mechanism as the bladder expands.
Renal scarring in VUR mainly occurs via postinflammatory fibrosis due to infection and mechanical pressure. Infected urine refluxing to the kidney generates an inflammatory response that often results in fibrosis and subsequent cortical scarring. This is most prevalent in patients with grades III to V VUR. Additionally, severe intrarenal reflux of sterile urine can elevate urinary mechanical back pressure within the renal pelvis and tubules through the "water hammer effect." Consequently, this can cause tubular destruction, parenchymal atrophy, and cortical scarring.[26][233]
Ultrasound is typically the preferred diagnostic method for initially detecting renal cortical abnormalities. Nuclear renal scanning with dimercaptosuccinic acid provides a more accurate assessment of renal scarring, although it involves radiation exposure and necessitates special equipment and IV access. VCUG remains the preferred radiographic examination for diagnosing VUR grade and identifying anatomical details.[234][235]
Direct radionuclide cystography is an alternative for diagnosing VUR but does not provide detailed anatomical information. The radiation burden of this modality is quite similar to that of VCUG when the study uses modern dose reduction techniques.[236] Contrast-enhanced ultrasonography is another modality for diagnosing VUR and is also useful in diagnosing urethral pathology. The main advantage of contrast-enhanced ultrasonography is that the modality prevents patient radiation exposure.[237]
The presence of VUR during the filling phase of the VCUG indicates a higher grade of reflux, a lower probability of spontaneous resolution, and an increased likelihood of requiring corrective surgery. The ureteral diameter ratio, an objective mathematical measure, seems to be more predictive of clinical outcomes compared to VUR grade.[238] Studies have indicated that a higher VUR index and ureteral diameter ratio exhibit greater predictive value than reflux grade for patients experiencing breakthrough UTIs with VUR.[165][239]
Medical Management of Vesicoureteric Reflux
The management of VUR has evolved and depends on the presence and frequency or absence of febrile UTIs, the age and phenotypic sex of the patient, the presence of bladder or bowel dysfunction, and the VUR grade. Spontaneous resolution of VUR frequently occurs before age 5, especially in younger patients, males, and those with lower VUR grades (I-III), making surgical intervention unnecessary in uncomplicated cases.[232][240]
Continuous antibiotic prophylaxis is often used to prevent UTIs and reduce the risk of renal damage associated with VUR. In infants with VUR and a febrile UTI, the morbidity is notably high, prompting the recommendation for continuous antibiotic prophylaxis regardless of VUR grade. Similarly, prophylaxis is recommended for high-grade VUR (grades III-V) regardless of UTI history. Nitrofurantoin, cephalosporins, and trimethoprim or sulfamethoxazole are commonly used drugs of choice for prophylaxis. However, there are discrepancies in the use of prophylaxis for VUR grades I and II without a history of febrile UTIs.[241]
Some studies caution against the routine use of prophylaxis due to its limited effectiveness in preventing renal damage and its potential to contribute to antibiotic resistance. However, other research suggests maintaining prophylaxis for at least 1 year and performing a VCUG annually. The risk of UTIs is higher in females and uncircumcised males. Before discontinuing prophylaxis, a renal scan is recommended to detect any cortical abnormalities or kidney scarring. Surgical intervention should be considered for patients experiencing recurrent UTIs, progressive renal damage, additional scarring, or inadequate kidney growth despite continued prophylaxis.
Surgical Management of Vesicoureteric Reflux
Surgery is considered the preferred treatment for patients with VUR and high-grade reflux, those with a low likelihood of spontaneous resolution, evidence of renal damage, or recurrent UTIs despite continuous antibiotic prophylaxis.[242][243] Multiple surgical techniques are available, each with its own set of risks and benefits. While traditional surgery via laparotomy is still widely regarded as the gold standard for managing VUR surgically, minimally invasive procedures such as robotically assisted surgeries and endoscopic periureteral bulking treatments have become increasingly prevalent and generally yield favorable outcomes.
Enhancing Healthcare Team Outcomes
VCUG is the gold-standard procedure for diagnosing VUR, necessitating seamless interprofessional communication among urologists, surgeons, and radiologists to tailor the study to each patient's needs. Successful VCUG execution requires precise coordination among nurses, pharmacists, radiology technologists, and radiologists to ensure atraumatic catheter placement, accurate contrast administration, and optimal imaging timing. Before the procedure, the nurse, radiologist, or technologist provides detailed explanations to the patient or parents to alleviate any anxiety or concerns. This counseling and clear communication help minimize discomfort and apprehension, particularly in children.[244] In addition, sedation with proper monitoring and support should be readily available to help calm any anxious child if necessary.
The combined responsibility of all involved personnel is to minimize radiation exposure by adhering to the "ALARA" principle. This involves implementing several measures to reduce patient radiation exposure during fluoroscopy.[245] Notably, it is crucial for the entire healthcare team to inform the patient, family, and parents about VUR comprehensively. This includes discussing the necessity for treatment, understanding the disorder's natural history, the potential consequences of untreated VUR, and the available treatment options, including surgical interventions. Recommendations should encompass an evaluation of the patient's likelihood of adhering to the treatment plan and complying with necessary follow-up examinations. In addition, it is important to establish criteria for determining when additional therapy may be necessary. When choosing between treatment options with similar risk or benefit profiles, parental concerns and preferences should be taken into careful consideration.
Media
(Click Image to Enlarge)
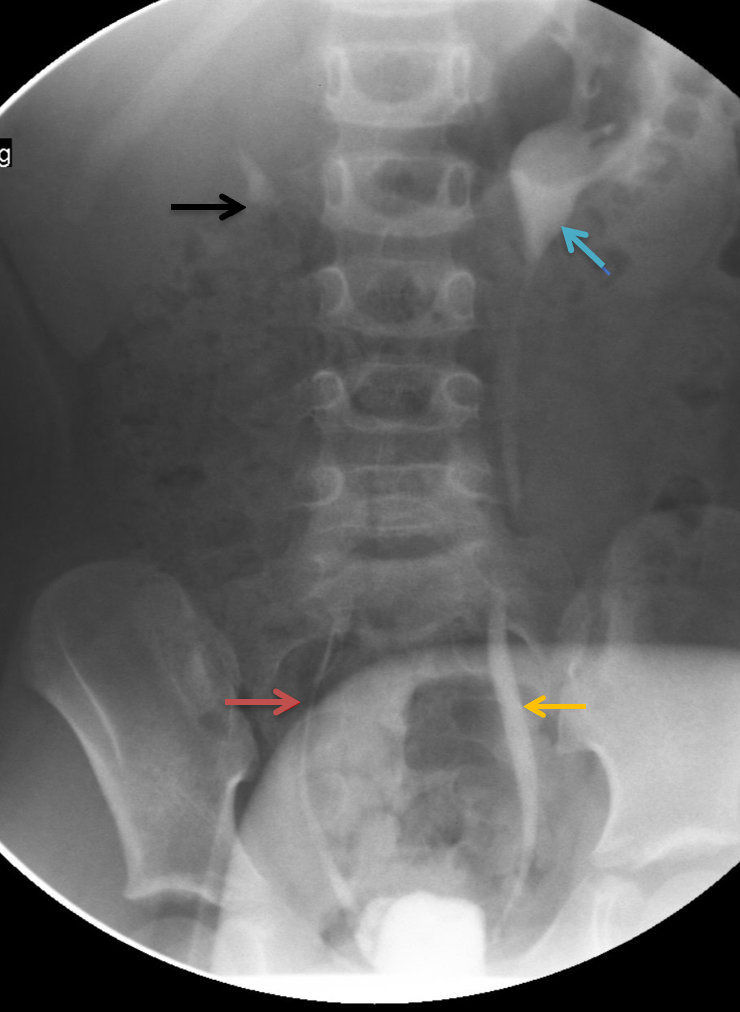
Fluoroscopic Spot Image Revealing Bilateral VUR on VCUG. The fluoroscopic spot image captures a voiding cystourethrogram (VCUG), revealing bilateral vesicoureteral reflux (VUR). Grade II VUR is observed on the right side, with contrast refluxing into the non-dilated ureter (red arrow) and pyelocalyceal system (black arrow) without significant dilatation. Grade III VUR is evident on the left side, with contrast refluxing into the mildly dilated ureter (yellow arrow) and mildly dilated left renal pelvis (blue arrow).
Le Bonheur Children's Hospital
References
HUTCH JA, BUNGE RG, FLOCKS RH. Vesicoureteral reflux in children. The Journal of urology. 1955 Nov:74(5):607-20 [PubMed PMID: 13272272]
HODSON CJ, EDWARDS D. Chronic pyelonephritis and vesico-ureteric reflex. Clinical radiology. 1960 Oct:11():219-31 [PubMed PMID: 13714877]
Hiraoka M, Hori C, Tsukahara H, Kasuga K, Ishihara Y, Kotsuji F, Mayumi M. Vesicoureteral reflux in male and female neonates as detected by voiding ultrasonography. Kidney international. 1999 Apr:55(4):1486-90 [PubMed PMID: 10201014]
van Eerde AM, Meutgeert MH, de Jong TP, Giltay JC. Vesico-ureteral reflux in children with prenatally detected hydronephrosis: a systematic review. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2007 Apr:29(4):463-9 [PubMed PMID: 17390310]
Level 1 (high-level) evidenceHoberman A, Charron M, Hickey RW, Baskin M, Kearney DH, Wald ER. Imaging studies after a first febrile urinary tract infection in young children. The New England journal of medicine. 2003 Jan 16:348(3):195-202 [PubMed PMID: 12529459]
Level 1 (high-level) evidenceMattoo TK. Vesicoureteral reflux and reflux nephropathy. Advances in chronic kidney disease. 2011 Sep:18(5):348-54. doi: 10.1053/j.ackd.2011.07.006. Epub [PubMed PMID: 21896376]
Level 3 (low-level) evidenceBourchier D, Abbott GD, Maling TM. Radiological abnormalities in infants with urinary tract infections. Archives of disease in childhood. 1984 Jul:59(7):620-4 [PubMed PMID: 6465930]
Sargent MA, Stringer DA. Voiding cystourethrography in children with urinary tract infection: the frequency of vesicoureteric reflux is independent of the specialty of the physician requesting the study. AJR. American journal of roentgenology. 1995 May:164(5):1237-41 [PubMed PMID: 7717238]
Level 2 (mid-level) evidenceSkoog SJ, Peters CA, Arant BS Jr, Copp HL, Elder JS, Hudson RG, Khoury AE, Lorenzo AJ, Pohl HG, Shapiro E, Snodgrass WT, Diaz M. Pediatric Vesicoureteral Reflux Guidelines Panel Summary Report: Clinical Practice Guidelines for Screening Siblings of Children With Vesicoureteral Reflux and Neonates/Infants With Prenatal Hydronephrosis. The Journal of urology. 2010 Sep:184(3):1145-51. doi: 10.1016/j.juro.2010.05.066. Epub 2010 Jul 21 [PubMed PMID: 20650494]
Level 1 (high-level) evidenceKaefer M, Curran M, Treves ST, Bauer S, Hendren WH, Peters CA, Atala A, Diamond D, Retik A. Sibling vesicoureteral reflux in multiple gestation births. Pediatrics. 2000 Apr:105(4 Pt 1):800-4 [PubMed PMID: 10742323]
Noe HN. The long-term results of prospective sibling reflux screening. The Journal of urology. 1992 Nov:148(5 Pt 2):1739-42 [PubMed PMID: 1433599]
Wan J, Greenfield SP, Ng M, Zerin M, Ritchey ML, Bloom D. Sibling reflux: a dual center retrospective study. The Journal of urology. 1996 Aug:156(2 Pt 2):677-9 [PubMed PMID: 8683758]
Level 2 (mid-level) evidenceChertin B, Puri P. Familial vesicoureteral reflux. The Journal of urology. 2003 May:169(5):1804-8 [PubMed PMID: 12686848]
McLorie GA, Sheldon CA, Fleisher M, Churchill BM. The genitourinary system in patients with imperforate anus. Journal of pediatric surgery. 1987 Dec:22(12):1100-4 [PubMed PMID: 3440894]
Sanchez S, Ricca R, Joyner B, Waldhausen JH. Vesicoureteral reflux and febrile urinary tract infections in anorectal malformations: a retrospective review. Journal of pediatric surgery. 2014 Jan:49(1):91-4; discussion 94. doi: 10.1016/j.jpedsurg.2013.09.031. Epub 2013 Oct 5 [PubMed PMID: 24439588]
Level 2 (mid-level) evidenceDeFoor W, Minevich E, Tackett L, Yasar U, Wacksman J, Sheldon C. Ectopic ureterocele: clinical application of classification based on renal unit jeopardy. The Journal of urology. 2003 Mar:169(3):1092-4 [PubMed PMID: 12576859]
Level 2 (mid-level) evidenceSalvatierra O Jr, Kountz SL, Belzer FO. Primary vesicoureteral reflux and end-stage renal disease. JAMA. 1973 Dec 17:226(12):1454-6 [PubMed PMID: 4800812]
Keren R, Shaikh N, Pohl H, Gravens-Mueller L, Ivanova A, Zaoutis L, Patel M, deBerardinis R, Parker A, Bhatnagar S, Haralam MA, Pope M, Kearney D, Sprague B, Barrera R, Viteri B, Egigueron M, Shah N, Hoberman A. Risk Factors for Recurrent Urinary Tract Infection and Renal Scarring. Pediatrics. 2015 Jul:136(1):e13-21. doi: 10.1542/peds.2015-0409. Epub 2015 Jun 8 [PubMed PMID: 26055855]
Shaikh N, Hoberman A, Keren R, Gotman N, Docimo SG, Mathews R, Bhatnagar S, Ivanova A, Mattoo TK, Moxey-Mims M, Carpenter MA, Pohl HG, Greenfield S. Recurrent Urinary Tract Infections in Children With Bladder and Bowel Dysfunction. Pediatrics. 2016 Jan:137(1):. doi: 10.1542/peds.2015-2982. Epub 2015 Dec 8 [PubMed PMID: 26647376]
Sjöström S, Sillén U, Bachelard M, Johansson E, Brandström P, Hellström AL, Abrahamsson K. Bladder/bowel dysfunction in pre-school children following febrile urinary tract infection in infancy. Pediatric nephrology (Berlin, Germany). 2021 Jun:36(6):1489-1497. doi: 10.1007/s00467-020-04853-4. Epub 2020 Dec 4 [PubMed PMID: 33274398]
Elder JS, Diaz M. Vesicoureteral reflux--the role of bladder and bowel dysfunction. Nature reviews. Urology. 2013 Nov:10(11):640-8. doi: 10.1038/nrurol.2013.221. Epub 2013 Oct 15 [PubMed PMID: 24126731]
Level 3 (low-level) evidenceUpadhyay J, Bolduc S, Bagli DJ, McLorie GA, Khoury AE, Farhat W. Use of the dysfunctional voiding symptom score to predict resolution of vesicoureteral reflux in children with voiding dysfunction. The Journal of urology. 2003 May:169(5):1842-6; discussion 1846; author reply 1846 [PubMed PMID: 12686859]
Koff SA, Wagner TT, Jayanthi VR. The relationship among dysfunctional elimination syndromes, primary vesicoureteral reflux and urinary tract infections in children. The Journal of urology. 1998 Sep:160(3 Pt 2):1019-22 [PubMed PMID: 9719268]
Kobayashi Y, Mishina H, Michihata N, Miyasaka M, Takayama JI. Indication for voiding cystourethrography during first urinary tract infection. Pediatrics international : official journal of the Japan Pediatric Society. 2019 Jun:61(6):595-600. doi: 10.1111/ped.13835. Epub [PubMed PMID: 30888085]
Lebowitz RL. The detection and characterization of vesicoureteral reflux in the child. The Journal of urology. 1992 Nov:148(5 Pt 2):1640-2 [PubMed PMID: 1433579]
Lee LC, Lorenzo AJ, Koyle MA. The role of voiding cystourethrography in the investigation of children with urinary tract infections. Canadian Urological Association journal = Journal de l'Association des urologues du Canada. 2016 May-Jun:10(5-6):210-214 [PubMed PMID: 27713802]
Straus Takahashi M, Gustavo Ieiri Yamanari M, Henrique de Marqui Moraes P, Iglesias Lopes R, Chammas MC. Vesicoureteral reflux by contrast ultrasound, comparison with voiding and retrograde urethrocystography: A prospective accuracy study. Journal of pediatric urology. 2024 Feb:20(1):133.e1-133.e9. doi: 10.1016/j.jpurol.2023.10.014. Epub 2023 Oct 18 [PubMed PMID: 37925278]
Shaikh N, Spingarn RB, Hum SW. Dimercaptosuccinic acid scan or ultrasound in screening for vesicoureteral reflux among children with urinary tract infections. The Cochrane database of systematic reviews. 2016 Jul 5:7(7):CD010657. doi: 10.1002/14651858.CD010657.pub2. Epub 2016 Jul 5 [PubMed PMID: 27378557]
Level 1 (high-level) evidencePaltiel HJ, Rupich RC, Kiruluta HG. Enhanced detection of vesicoureteral reflux in infants and children with use of cyclic voiding cystourethrography. Radiology. 1992 Sep:184(3):753-5 [PubMed PMID: 1509062]
Arlen AM, Amin J, Leong T. Voiding cystourethrogram: Who gets a cyclic study and does it matter? Journal of pediatric urology. 2022 Jun:18(3):378-382. doi: 10.1016/j.jpurol.2022.02.008. Epub 2022 Feb 17 [PubMed PMID: 35241383]
Frimberger D, Mercado-Deane MG, SECTION ON UROLOGY, SECTION ON RADIOLOGY. Establishing a Standard Protocol for the Voiding Cystourethrography. Pediatrics. 2016 Nov:138(5):. pii: e20162590. Epub [PubMed PMID: 27940792]
Lebowitz RL, Olbing H, Parkkulainen KV, Smellie JM, Tamminen-Möbius TE. International system of radiographic grading of vesicoureteric reflux. International Reflux Study in Children. Pediatric radiology. 1985:15(2):105-9 [PubMed PMID: 3975102]
. Medical versus surgical treatment of primary vesicoureteral reflux: report of the International Reflux Study Committee. Pediatrics. 1981 Mar:67(3):392-400 [PubMed PMID: 7017578]
Lebowitz RL, Blickman JG. The coexistence of ureteropelvic junction obstruction and reflux. AJR. American journal of roentgenology. 1983 Feb:140(2):231-8 [PubMed PMID: 6600335]
Level 3 (low-level) evidencePapadopoulou F, Efremidis SC, Oiconomou A, Badouraki M, Panteleli M, Papachristou F, Soteriou I. Cyclic voiding cystourethrography: is vesicoureteral reflux missed with standard voiding cystourethrography? European radiology. 2002 Mar:12(3):666-70 [PubMed PMID: 11870484]
Darge K, Troeger J. Vesicoureteral reflux grading in contrast-enhanced voiding urosonography. European journal of radiology. 2002 Aug:43(2):122-8 [PubMed PMID: 12127209]
Roic AC, Milošević D, Turudić D, Roic G. An innovative diagnostic procedure in children: videourodynamics with contrast-enhanced voiding urosonography. Journal of ultrasound. 2023 Jun:26(2):583-587. doi: 10.1007/s40477-022-00721-z. Epub 2022 Nov 22 [PubMed PMID: 36417175]
Benya EC. Contrast-enhanced voiding urosonography for video urodynamics: feasible but not perfect. Pediatric radiology. 2023 Jul:53(8):1720-1721. doi: 10.1007/s00247-023-05634-5. Epub 2023 Mar 3 [PubMed PMID: 36867205]
Sofia C, Solazzo A, Cattafi A, Chimenz R, Cicero G, Marino MA, D'angelo T, Manti L, Condorelli E, Ceravolo G, Mazziotti S, Ascenti G. Contrast-enhanced voiding urosonography in the assessment of vesical-ureteral reflux: the time has come. La Radiologia medica. 2021 Jul:126(7):901-909. doi: 10.1007/s11547-021-01360-w. Epub 2021 May 5 [PubMed PMID: 33954899]
Ntoulia A, Aguirre Pascual E, Back SJ, Bellah RD, Beltrán Salazar VP, Chan PKJ, Chow JS, Coca Robinot D, Darge K, Duran C, Epelman M, Ključevšek D, Kwon JK, Sandhu PK, Woźniak MM, Papadopoulou F. Contrast-enhanced voiding urosonography, part 1: vesicoureteral reflux evaluation. Pediatric radiology. 2021 Nov:51(12):2351-2367. doi: 10.1007/s00247-020-04906-8. Epub 2021 Mar 31 [PubMed PMID: 33787945]
Naseri M, Karimi M, Bakhtiari E, Tafazoli N, Alamdaran SA, Tafazoli N. Diagnostic Values of Kidney Ultrasonography for Vesicoureteral Reflux (VUR) and High Grade VUR. Iranian journal of kidney diseases. 2021 Sep:15(5):328-335 [PubMed PMID: 34582367]
Likartsis C, Printza N, Notopoulos A. Radionuclide techniques for the detection of vesicoureteral reflux and their clinical significance. Hellenic journal of nuclear medicine. 2020 May-Aug:23(2):180-187. doi: 10.1967/s002449912107. Epub 2020 Jul 27 [PubMed PMID: 32716409]
Torun N, Aktas A, Reyhan M, Yapar AF, Nursal GN. Evaluation of cyclic direct radionuclide cystography findings with DMSA scintigraphy results in children with a prior diagnosis of vesicoureteral reflux. Nuclear medicine communications. 2019 Jun:40(6):583-587. doi: 10.1097/MNM.0000000000000994. Epub [PubMed PMID: 30741838]
D'Errico G. The role of nuclear medicine in evaluation of vesicoureteral reflux and/or reflux nephropathy. Rays. 2002 Apr-Jun:27(2):149-54 [PubMed PMID: 12696269]
Ergun R, Sekerci CA, Tanidir Y, Telli O, Kutukoglu MU, Tarcan T, Yucel S. Abnormal DMSA renal scan findings and associated factors in older children with vesicoureteral reflux. International urology and nephrology. 2021 Oct:53(10):1963-1968. doi: 10.1007/s11255-021-02934-3. Epub 2021 Jul 2 [PubMed PMID: 34213712]
Cajigas-Loyola SC, Chow JS, Hayatghaibi S, Iyer RS, Kwon J, Rubesova E, Sánchez-Jacob R, Wyers M, Otero HJ. Imaging of Vesicoureteral Reflux: AJR Expert Panel Narrative Review. AJR. American journal of roentgenology. 2023 Sep 6:():. doi: 10.2214/AJR.23.29741. Epub 2023 Sep 6 [PubMed PMID: 37672329]
Level 3 (low-level) evidenceHari P, Meena J, Kumar M, Sinha A, Thergaonkar RW, Iyengar A, Khandelwal P, Ekambaram S, Pais P, Sharma J, Kanitkar M, Bagga A, Indian Society of Pediatric Nephrology. Evidence-based clinical practice guideline for management of urinary tract infection and primary vesicoureteric reflux. Pediatric nephrology (Berlin, Germany). 2024 May:39(5):1639-1668. doi: 10.1007/s00467-023-06173-9. Epub 2023 Oct 28 [PubMed PMID: 37897526]
Level 1 (high-level) evidenceTsai JD, Huang CT, Lin PY, Chang JH, Lee MD, Huang FY, Shih BF, Hung HY, Hsu CH, Kao HA, Lin CC. Screening high-grade vesicoureteral reflux in young infants with a febrile urinary tract infection. Pediatric nephrology (Berlin, Germany). 2012 Jun:27(6):955-63. doi: 10.1007/s00467-012-2104-1. Epub 2012 Mar 1 [PubMed PMID: 22374404]
Sheu JN, Wu KH, Chen SM, Tsai JD, Chao YH, Lue KH. Acute 99mTc DMSA scan predicts dilating vesicoureteral reflux in young children with a first febrile urinary tract infection: a population-based cohort study. Clinical nuclear medicine. 2013 Mar:38(3):163-8. doi: 10.1097/RLU.0b013e318279f112. Epub [PubMed PMID: 23354031]
Level 2 (mid-level) evidenceHollowell JG, Greenfield SP. Screening siblings for vesicoureteral reflux. The Journal of urology. 2002 Nov:168(5):2138-41 [PubMed PMID: 12394743]
Menezes M, Puri P. Familial vesicoureteral reflux--is screening beneficial? The Journal of urology. 2009 Oct:182(4 Suppl):1673-7. doi: 10.1016/j.juro.2009.02.087. Epub 2009 Aug 19 [PubMed PMID: 19692047]
Nelson CP, Finkelstein JA, Logvinenko T, Schuster MA. Incidence of Urinary Tract Infection Among Siblings of Children With Vesicoureteral Reflux. Academic pediatrics. 2016 Jul:16(5):489-495. doi: 10.1016/j.acap.2015.11.003. Epub 2015 Nov 14 [PubMed PMID: 26589543]
Onen A. Grading of Hydronephrosis: An Ongoing Challenge. Frontiers in pediatrics. 2020:8():458. doi: 10.3389/fped.2020.00458. Epub 2020 Aug 27 [PubMed PMID: 32984198]
Rickard M, Easterbrook B, Kim S, Farrokhyar F, Stein N, Arora S, Belostotsky V, DeMaria J, Lorenzo AJ, Braga LH. Six of one, half a dozen of the other: A measure of multidisciplinary inter/intra-rater reliability of the society for fetal urology and urinary tract dilation grading systems for hydronephrosis. Journal of pediatric urology. 2017 Feb:13(1):80.e1-80.e5. doi: 10.1016/j.jpurol.2016.09.005. Epub 2016 Oct 27 [PubMed PMID: 27916387]
Han M, Kim HG, Lee JD, Park SY, Sur YK. Conversion and reliability of two urological grading systems in infants: the Society for Fetal Urology and the urinary tract dilatation classifications system. Pediatric radiology. 2017 Jan:47(1):65-73. doi: 10.1007/s00247-016-3721-9. Epub 2016 Oct 10 [PubMed PMID: 27725992]
Hwang J, Kim PH, Yoon HM, Song SH, Jung AY, Lee JS, Cho YA. Application of the postnatal urinary tract dilation classification system to predict the need for surgical intervention among neonates and young infants. Ultrasonography (Seoul, Korea). 2023 Jan:42(1):136-146. doi: 10.14366/usg.22035. Epub 2022 Jul 23 [PubMed PMID: 36464956]
Ellison JS, Dy GW, Fu BC, Holt SK, Gore JL, Merguerian PA. Neonatal Circumcision and Urinary Tract Infections in Infants With Hydronephrosis. Pediatrics. 2018 Jul:142(1):. pii: e20173703. doi: 10.1542/peds.2017-3703. Epub 2018 Jun 7 [PubMed PMID: 29880703]
Wahyudi I, Raharja PAR, Situmorang GR, Rodjani A. Circumcision reduces urinary tract infection in children with antenatal hydronephrosis: Systematic review and meta-analysis. Journal of pediatric urology. 2023 Feb:19(1):66-74. doi: 10.1016/j.jpurol.2022.10.029. Epub 2022 Oct 28 [PubMed PMID: 36371332]
Level 1 (high-level) evidenceKose E, Yavascan O, Turan O, Kangin M, Bal A, Alparslan C, Sirin Kose S, Kuyum P, Aksu N. The effect of circumcision on the frequency of urinary tract infection, growth and nutrition status in infants with antenatal hydronephrosis. Renal failure. 2013:35(10):1365-9. doi: 10.3109/0886022X.2013.828263. Epub 2013 Sep 2 [PubMed PMID: 23992538]
Zareba P, Lorenzo AJ, Braga LH. Risk factors for febrile urinary tract infection in infants with prenatal hydronephrosis: comprehensive single center analysis. The Journal of urology. 2014 May:191(5 Suppl):1614-8. doi: 10.1016/j.juro.2013.10.035. Epub 2014 Mar 26 [PubMed PMID: 24679866]
Herthelius M. Antenatally detected urinary tract dilatation: long-term outcome. Pediatric nephrology (Berlin, Germany). 2023 Oct:38(10):3221-3227. doi: 10.1007/s00467-023-05907-z. Epub 2023 Mar 15 [PubMed PMID: 36920569]
Santos JD, Lopes RI, Koyle MA. Bladder and bowel dysfunction in children: An update on the diagnosis and treatment of a common, but underdiagnosed pediatric problem. Canadian Urological Association journal = Journal de l'Association des urologues du Canada. 2017 Jan-Feb:11(1-2Suppl1):S64-S72. doi: 10.5489/cuaj.4411. Epub [PubMed PMID: 28265323]
Garin EH. Primary vesicoureteral reflux; what have we learnt from the recently published randomized, controlled trials? Pediatric nephrology (Berlin, Germany). 2019 Sep:34(9):1513-1519. doi: 10.1007/s00467-018-4045-9. Epub 2018 Aug 21 [PubMed PMID: 30132079]
Level 1 (high-level) evidenceEscolino M, Kalfa N, Castagnetti M, Caione P, Esposito G, Florio L, Esposito C. Endoscopic injection of bulking agents in pediatric vesicoureteral reflux: a narrative review of the literature. Pediatric surgery international. 2023 Feb 18:39(1):133. doi: 10.1007/s00383-023-05426-w. Epub 2023 Feb 18 [PubMed PMID: 36806763]
Level 3 (low-level) evidenceEstrada CR Jr, Passerotti CC, Graham DA, Peters CA, Bauer SB, Diamond DA, Cilento BG Jr, Borer JG, Cendron M, Nelson CP, Lee RS, Zhou J, Retik AB, Nguyen HT. Nomograms for predicting annual resolution rate of primary vesicoureteral reflux: results from 2,462 children. The Journal of urology. 2009 Oct:182(4):1535-41. doi: 10.1016/j.juro.2009.06.053. Epub 2009 Aug 15 [PubMed PMID: 19683762]
Elder JS, Peters CA, Arant BS Jr, Ewalt DH, Hawtrey CE, Hurwitz RS, Parrott TS, Snyder HM 3rd, Weiss RA, Woolf SH, Hasselblad V. Pediatric Vesicoureteral Reflux Guidelines Panel summary report on the management of primary vesicoureteral reflux in children. The Journal of urology. 1997 May:157(5):1846-51 [PubMed PMID: 9112544]
Level 1 (high-level) evidenceGodley ML, Desai D, Yeung CK, Dhillon HK, Duffy PG, Ransley PG. The relationship between early renal status, and the resolution of vesico-ureteric reflux and bladder function at 16 months. BJU international. 2001 Apr:87(6):457-62 [PubMed PMID: 11298034]
Sjöström S, Sillén U, Bachelard M, Hansson S, Stokland E. Spontaneous resolution of high grade infantile vesicoureteral reflux. The Journal of urology. 2004 Aug:172(2):694-8; discussion 699 [PubMed PMID: 15247764]
Rösch W. [Anti-reflux surgery in infancy: contra]. Aktuelle Urologie. 2020 Apr:51(2):165-170. doi: 10.1055/a-1026-2822. Epub 2019 Dec 3 [PubMed PMID: 31797339]
Zhao B, Ivanova A, Shaikh N. Antimicrobial prophylaxis for vesicoureteral reflux: which subgroups of children benefit the most? Research square. 2023 Aug 30:():. pii: rs.3.rs-3286108. doi: 10.21203/rs.3.rs-3286108/v1. Epub 2023 Aug 30 [PubMed PMID: 37693511]
Morello W, Baskin E, Jankauskiene A, Yalcinkaya F, Zurowska A, Puccio G, Serafinelli J, La Manna A, Krzemień G, Pennesi M, La Scola C, Becherucci F, Brugnara M, Yuksel S, Mekahli D, Chimenz R, De Palma D, Zucchetta P, Vajauskas D, Drozdz D, Szczepanska M, Caliskan S, Lombet J, Minoli DG, Guarino S, Gulleroglu K, Ruzgiene D, Szmigielska A, Barbi E, Ozcakar ZB, Kranz A, Pasini A, Materassi M, De Rechter S, Ariceta G, Weber LT, Marzuillo P, Alberici I, Taranta-Janusz K, Caldas Afonso A, Tkaczyk M, Català M, Cabrera Sevilla JE, Mehls O, Schaefer F, Montini G, PREDICT Study Group. Antibiotic Prophylaxis in Infants with Grade III, IV, or V Vesicoureteral Reflux. The New England journal of medicine. 2023 Sep 14:389(11):987-997. doi: 10.1056/NEJMoa2300161. Epub 2023 Sep 12 [PubMed PMID: 37702442]
Nakamura M, Moriya K, Kon M, Nishimura Y, Chiba H, Kitta T, Shinohara N. Girls and renal scarring as risk factors for febrile urinary tract infection after stopping antibiotic prophylaxis in children with vesicoureteral reflux. World journal of urology. 2021 Jul:39(7):2587-2595. doi: 10.1007/s00345-020-03524-1. Epub 2021 Jan 3 [PubMed PMID: 33388912]
Horsager TH, Hagstrøm S, Skals R, Winding L. Renal scars in children with febrile urinary tract infection - Looking for associated factors. Journal of pediatric urology. 2022 Oct:18(5):682.e1-682.e9. doi: 10.1016/j.jpurol.2022.09.012. Epub 2022 Sep 17 [PubMed PMID: 36253233]
Yılmaz S, Özçakar ZB, Kurt Şükür ED, Bulum B, Kavaz A, Elhan AH, Yalçınkaya F. Vesicoureteral Reflux and Renal Scarring Risk in Children after the First Febrile Urinary Tract Infection. Nephron. 2016:132(3):175-80. doi: 10.1159/000443536. Epub 2016 Feb 23 [PubMed PMID: 26901769]
Hari P, Hari S, Sinha A, Kumar R, Kapil A, Pandey RM, Bagga A. Antibiotic prophylaxis in the management of vesicoureteric reflux: a randomized double-blind placebo-controlled trial. Pediatric nephrology (Berlin, Germany). 2015 Mar:30(3):479-86. doi: 10.1007/s00467-014-2943-z. Epub 2014 Aug 31 [PubMed PMID: 25173357]
Level 1 (high-level) evidencePeters CA, Skoog SJ, Arant BS Jr, Copp HL, Elder JS, Hudson RG, Khoury AE, Lorenzo AJ, Pohl HG, Shapiro E, Snodgrass WT, Diaz M. Summary of the AUA Guideline on Management of Primary Vesicoureteral Reflux in Children. The Journal of urology. 2010 Sep:184(3):1134-44. doi: 10.1016/j.juro.2010.05.065. Epub 2010 Jul 21 [PubMed PMID: 20650499]
Williams GJ, Wei L, Lee A, Craig JC. Long-term antibiotics for preventing recurrent urinary tract infection in children. The Cochrane database of systematic reviews. 2006 Jul 19:(3):CD001534 [PubMed PMID: 16855971]
Level 1 (high-level) evidenceRasmussen SH, Shrestha S, Bjerregaard LG, Ängquist LH, Baker JL, Jess T, Allin KH. Antibiotic exposure in early life and childhood overweight and obesity: A systematic review and meta-analysis. Diabetes, obesity & metabolism. 2018 Jun:20(6):1508-1514. doi: 10.1111/dom.13230. Epub 2018 Feb 25 [PubMed PMID: 29359849]
Level 1 (high-level) evidenceMiller SA, Wu RKS, Oremus M. The association between antibiotic use in infancy and childhood overweight or obesity: a systematic review and meta-analysis. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2018 Nov:19(11):1463-1475. doi: 10.1111/obr.12717. Epub 2018 Jul 23 [PubMed PMID: 30035851]
Level 1 (high-level) evidenceAghaali M, Hashemi-Nazari SS. Association between early antibiotic exposure and risk of childhood weight gain and obesity: a systematic review and meta-analysis. Journal of pediatric endocrinology & metabolism : JPEM. 2019 May 27:32(5):439-445. doi: 10.1515/jpem-2018-0437. Epub [PubMed PMID: 31042643]
Level 1 (high-level) evidenceMeng X, Zhu Y, Di H, Zhang M, Feng J, Xu M, Xia W, Tian Q, He Y, Gan Y, Lu Z. Dose-response association of early-life antibiotic exposure and subsequent overweight or obesity in children: A meta-analysis of prospective studies. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2021 Nov:22(11):e13321. doi: 10.1111/obr.13321. Epub 2021 Jul 29 [PubMed PMID: 34328260]
Level 1 (high-level) evidenceSchwartz DJ, Langdon AE, Dantas G. Understanding the impact of antibiotic perturbation on the human microbiome. Genome medicine. 2020 Sep 28:12(1):82. doi: 10.1186/s13073-020-00782-x. Epub 2020 Sep 28 [PubMed PMID: 32988391]
Level 3 (low-level) evidenceRamirez J, Guarner F, Bustos Fernandez L, Maruy A, Sdepanian VL, Cohen H. Antibiotics as Major Disruptors of Gut Microbiota. Frontiers in cellular and infection microbiology. 2020:10():572912. doi: 10.3389/fcimb.2020.572912. Epub 2020 Nov 24 [PubMed PMID: 33330122]
Autore G, Bernardi L, Ghidini F, La Scola C, Berardi A, Biasucci G, Marchetti F, Pasini A, Capra ME, Castellini C, Cioni V, Cantatore S, Cella A, Cusenza F, De Fanti A, Della Casa Muttini E, Di Costanzo M, Dozza A, Gatti C, Malaventura C, Pierantoni L, Parente G, Pelusi G, Perrone S, Serra L, Torcetta F, Valletta E, Vergine G, Antodaro F, Bergomi A, Chiarlolanza J, Leoni L, Mazzini F, Sacchetti R, Suppiej A, Iughetti L, Pession A, Lima M, Esposito S, The Uti-Ped-Er Study Group. Antibiotic Prophylaxis for the Prevention of Urinary Tract Infections in Children: Guideline and Recommendations from the Emilia-Romagna Pediatric Urinary Tract Infections (UTI-Ped-ER) Study Group. Antibiotics (Basel, Switzerland). 2023 Jun 12:12(6):. doi: 10.3390/antibiotics12061040. Epub 2023 Jun 12 [PubMed PMID: 37370359]
Selekman RE, Shapiro DJ, Boscardin J, Williams G, Craig JC, Brandström P, Pennesi M, Roussey-Kesler G, Hari P, Copp HL. Uropathogen Resistance and Antibiotic Prophylaxis: A Meta-analysis. Pediatrics. 2018 Jul:142(1):. doi: 10.1542/peds.2018-0119. Epub [PubMed PMID: 29954832]
Level 1 (high-level) evidenceSubcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management, Roberts KB. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011 Sep:128(3):595-610. doi: 10.1542/peds.2011-1330. Epub 2011 Aug 28 [PubMed PMID: 21873693]
Level 1 (high-level) evidenceNagler EV, Williams G, Hodson EM, Craig JC. Interventions for primary vesicoureteric reflux. The Cochrane database of systematic reviews. 2011 Jun 15:(6):CD001532. doi: 10.1002/14651858.CD001532.pub4. Epub 2011 Jun 15 [PubMed PMID: 21678334]
Level 1 (high-level) evidenceNagler EV. Primary vesicoureteric reflux grade I to V: no compelling evidence for routine antibiotics or surgical intervention. Journal of paediatrics and child health. 2013 Oct:49(10):876-9. doi: 10.1111/jpc.12383. Epub [PubMed PMID: 24131127]
Bertsimas D, Li M, Estrada C, Nelson C, Scott Wang HH. Selecting Children with Vesicoureteral Reflux Who are Most Likely to Benefit from Antibiotic Prophylaxis: Application of Machine Learning to RIVUR. The Journal of urology. 2021 Apr:205(4):1170-1179. doi: 10.1097/JU.0000000000001445. Epub 2020 Dec 8 [PubMed PMID: 33289598]
Sunaryo PL, Cambareri GM, Winston DG, Hanna MK, Stock JA. Vesico-ureteric reflux (VUR) management and screening patterns: are paediatric urologists following the 2010 American Urological Association (AUA) guidelines? BJU international. 2014 Nov:114(5):761-9. doi: 10.1111/bju.12588. Epub 2014 Aug 16 [PubMed PMID: 24274826]
Gaither TW, Copp HL. Antimicrobial prophylaxis for urinary tract infections: implications for adherence assessment. Journal of pediatric urology. 2019 Aug:15(4):387.e1-387.e8. doi: 10.1016/j.jpurol.2019.04.019. Epub 2019 May 2 [PubMed PMID: 31182400]
Cheng CH, Tsai MH, Huang YC, Su LH, Tsau YK, Lin CJ, Chiu CH, Lin TY. Antibiotic resistance patterns of community-acquired urinary tract infections in children with vesicoureteral reflux receiving prophylactic antibiotic therapy. Pediatrics. 2008 Dec:122(6):1212-7. doi: 10.1542/peds.2007-2926. Epub [PubMed PMID: 19047236]
Level 2 (mid-level) evidenceKarpman E, Kurzrock EA. Adverse reactions of nitrofurantoin, trimethoprim and sulfamethoxazole in children. The Journal of urology. 2004 Aug:172(2):448-53 [PubMed PMID: 15247700]
Ortiz TK, Velazquez N, Ding L, Routh JC, Wiener JS, Seed PC, Ross SS. Predominant bacteria and patterns of antibiotic susceptibility in urinary tract infection in children with spina bifida. Journal of pediatric urology. 2018 Oct:14(5):444.e1-444.e8. doi: 10.1016/j.jpurol.2018.03.017. Epub 2018 Apr 20 [PubMed PMID: 29709445]
RIVUR Trial Investigators, Hoberman A, Greenfield SP, Mattoo TK, Keren R, Mathews R, Pohl HG, Kropp BP, Skoog SJ, Nelson CP, Moxey-Mims M, Chesney RW, Carpenter MA. Antimicrobial prophylaxis for children with vesicoureteral reflux. The New England journal of medicine. 2014 Jun 19:370(25):2367-76. doi: 10.1056/NEJMoa1401811. Epub 2014 May 4 [PubMed PMID: 24795142]
Level 1 (high-level) evidencePrát V, Konícková L, Hatala M, Urbanová D. [Attempted therapy of experimental ascending E. coli pyelonephritis by intravesical administration of furadantin]. Casopis lekaru ceskych. 1968:107(10):299-302 [PubMed PMID: 4874671]
Level 3 (low-level) evidenceKENNELLY BM. CANDIDA ALBICANS CYSTITIS CURED BY NYSTATIN BLADDER INSTILLATIONS. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 1965 May 22:39():414-7 [PubMed PMID: 14295761]
Defoor W, Ferguson D, Mashni S, Creelman L, Reeves D, Minevich E, Reddy P, Sheldon C. Safety of gentamicin bladder irrigations in complex urological cases. The Journal of urology. 2006 May:175(5):1861-4 [PubMed PMID: 16600780]
Level 2 (mid-level) evidenceHuen KH, Nik-Ahd F, Chen L, Lerman S, Singer J. Neomycin-polymyxin or gentamicin bladder instillations decrease symptomatic urinary tract infections in neurogenic bladder patients on clean intermittent catheterization. Journal of pediatric urology. 2019 Apr:15(2):178.e1-178.e7. doi: 10.1016/j.jpurol.2018.12.001. Epub 2018 Dec 12 [PubMed PMID: 30611650]
Cox L, He C, Bevins J, Clemens JQ, Stoffel JT, Cameron AP. Gentamicin bladder instillations decrease symptomatic urinary tract infections in neurogenic bladder patients on intermittent catheterization. Canadian Urological Association journal = Journal de l'Association des urologues du Canada. 2017 Sep:11(9):E350-E354. doi: 10.5489/cuaj.4434. Epub [PubMed PMID: 29382457]
van Merode NAM, Pat JJ, Wolfhagen MJHM, Dijkstra GA. Successfully treated bilateral renal fungal balls with continuous Anidulafulgin irrigation. Urology case reports. 2021 Jan:34():101468. doi: 10.1016/j.eucr.2020.101468. Epub 2020 Oct 26 [PubMed PMID: 33145176]
Level 3 (low-level) evidenceMouhssine M, Al Ani D, Al Shibli A, Ghatasheh G, Al Amri A, Matta H, Chedid R, Narchi H. Intravesical gentamicin instillation in the prevention of recurrent urinary tract infections in children with neurogenic bladder- a single-center retrospective observational study. Journal of pediatric urology. 2023 Feb:19(1):64.e1-64.e7. doi: 10.1016/j.jpurol.2022.09.001. Epub 2022 Sep 23 [PubMed PMID: 36216695]
Level 2 (mid-level) evidencevan Nieuwkoop C, den Exter PL, Elzevier HW, den Hartigh J, van Dissel JT. Intravesical gentamicin for recurrent urinary tract infection in patients with intermittent bladder catheterisation. International journal of antimicrobial agents. 2010 Dec:36(6):485-90. doi: 10.1016/j.ijantimicag.2010.05.005. Epub 2010 Jun 26 [PubMed PMID: 20580533]
Level 3 (low-level) evidenceBilsen MP, van Uhm JIM, Stalenhoef JE, van Nieuwkoop C, Groenwold RHH, Visser LG, Lambregts MMC. Intravesical aminoglycoside instillations as prophylaxis for recurrent urinary tract infection: patient satisfaction, long-term safety and efficacy. JAC-antimicrobial resistance. 2023 Apr:5(2):dlad040. doi: 10.1093/jacamr/dlad040. Epub 2023 Apr 6 [PubMed PMID: 37034119]
Ong A, Pietropaolo A, Brown G, Somani BK. Are Intravesical Aminoglycosides the New Gold Standard in the Management of Refractory Urinary Tract Infection: A Systematic Review of Literature. Journal of clinical medicine. 2022 Sep 27:11(19):. doi: 10.3390/jcm11195703. Epub 2022 Sep 27 [PubMed PMID: 36233570]
Level 1 (high-level) evidenceReddy M, Zimmern PE. Efficacy of antimicrobial intravesical treatment for uncomplicated recurrent urinary tract infections: a systematic review. International urogynecology journal. 2022 May:33(5):1125-1143. doi: 10.1007/s00192-021-05042-z. Epub 2022 Jan 4 [PubMed PMID: 34982189]
Level 1 (high-level) evidenceAndretta E, Longo R, Balladelli M, Sgarabotto C, Sgarabotto D. Intravesical Gentamicin: An Option for Therapy and Prophylaxis against Recurrent UTIs and Resistant Bacteria in Neurogenic Bladder Patients on Intermittent Catheterization. Antibiotics (Basel, Switzerland). 2022 Sep 30:11(10):. doi: 10.3390/antibiotics11101335. Epub 2022 Sep 30 [PubMed PMID: 36289993]
Morris CJ, Rohn JL, Glickman S, Mansfield KJ. Effective Treatments of UTI-Is Intravesical Therapy the Future? Pathogens (Basel, Switzerland). 2023 Mar 6:12(3):. doi: 10.3390/pathogens12030417. Epub 2023 Mar 6 [PubMed PMID: 36986339]
Pietropaolo A, Jones P, Moors M, Birch B, Somani BK. Use and Effectiveness of Antimicrobial Intravesical Treatment for Prophylaxis and Treatment of Recurrent Urinary Tract Infections (UTIs): a Systematic Review. Current urology reports. 2018 Aug 9:19(10):78. doi: 10.1007/s11934-018-0834-8. Epub 2018 Aug 9 [PubMed PMID: 30094687]
Level 1 (high-level) evidenceZiadeh T, Chebel R, Labaki C, Saliba G, Helou EE. Bladder instillation for urinary tract infection prevention in neurogenic bladder patients practicing clean intermittent catheterization: A systematic review. Urologia. 2022 May:89(2):261-267. doi: 10.1177/03915603211049883. Epub 2021 Oct 6 [PubMed PMID: 34612750]
Level 1 (high-level) evidenceDi Chiara C, Ponzoni M, Piché-Renaud PP, Mengato D, Giaquinto C, Morris SK, Donà D. Alternative Antimicrobial Irrigation Strategies for the Treatment of Infections in Children: A Review of the Existing Literature. Antibiotics (Basel, Switzerland). 2023 Aug 1:12(8):. doi: 10.3390/antibiotics12081271. Epub 2023 Aug 1 [PubMed PMID: 37627691]
Marei MM, Jackson R, Keene DJB. Intravesical gentamicin instillation for the treatment and prevention of urinary tract infections in complex paediatric urology patients: evidence for safety and efficacy. Journal of pediatric urology. 2021 Feb:17(1):65.e1-65.e11. doi: 10.1016/j.jpurol.2020.08.007. Epub 2020 Aug 19 [PubMed PMID: 33309610]
Abrams P, Hashim H, Tomson C, Macgowan A, Skews R, Warren K. The use of intravesical gentamicin to treat recurrent urinary tract infections in lower urinary tract dysfunction. Neurourology and urodynamics. 2017 Nov:36(8):2109-2116. doi: 10.1002/nau.23250. Epub 2017 May 15 [PubMed PMID: 28503891]
Wang ZT, Wehbi E, Alam Y, Khoury A. A Reanalysis of the RIVUR Trial Using a Risk Classification System. The Journal of urology. 2018 Jun:199(6):1608-1614. doi: 10.1016/j.juro.2017.11.080. Epub 2017 Dec 2 [PubMed PMID: 29198997]
Tewary K, Narchi H. Recurrent urinary tract infections in children: Preventive interventions other than prophylactic antibiotics. World journal of methodology. 2015 Jun 26:5(2):13-9. doi: 10.5662/wjm.v5.i2.13. Epub 2015 Jun 26 [PubMed PMID: 26140267]
Meena J, Thomas CC, Kumar J, Raut S, Hari P. Non-antibiotic interventions for prevention of urinary tract infections in children: a systematic review and meta-analysis of randomized controlled trials. European journal of pediatrics. 2021 Dec:180(12):3535-3545. doi: 10.1007/s00431-021-04091-2. Epub 2021 Jun 22 [PubMed PMID: 34156540]
Level 1 (high-level) evidenceWan KS, Liu CK, Lee WK, Ko MC, Huang CS. Cranberries for Preventing Recurrent Urinary Tract Infections in Uncircumcised Boys. Alternative therapies in health and medicine. 2016 Nov:22(6):20-23 [PubMed PMID: 27866177]
Hakkola M, Vehviläinen P, Muotka J, Tejesvi MV, Pokka T, Vähäsarja P, Hanni AM, Renko M, Uhari M, Salo J, Tapiainen T. Cranberry-lingonberry juice affects the gut and urinary microbiome in children - a randomized controlled trial. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica. 2023 Mar:131(3):112-124. doi: 10.1111/apm.13292. Epub 2023 Jan 18 [PubMed PMID: 36602283]
Level 1 (high-level) evidenceDurham SH, Stamm PL, Eiland LS. Cranberry Products for the Prophylaxis of Urinary Tract Infections in Pediatric Patients. The Annals of pharmacotherapy. 2015 Dec:49(12):1349-56. doi: 10.1177/1060028015606729. Epub 2015 Sep 23 [PubMed PMID: 26400007]
Saito Y, Yamanaka S, Fujimoto T, Tajiri S, Matsumoto N, Takamura T, Matsumoto K, Yokoo T. Mesangial cell regeneration from exogenous stromal progenitor by utilizing embryonic kidney. Biochemical and biophysical research communications. 2019 Dec 10:520(3):627-633. doi: 10.1016/j.bbrc.2019.10.080. Epub 2019 Oct 14 [PubMed PMID: 31623827]
Libretti S, Aeddula NR. Embryology, Genitourinary. StatPearls. 2024 Jan:(): [PubMed PMID: 32644735]
Yamanaka S. Generation of chimeric kidneys using progenitor cell replacement: Oshima Award Address 2021. Clinical and experimental nephrology. 2022 Jun:26(6):491-500. doi: 10.1007/s10157-022-02191-3. Epub 2022 Feb 9 [PubMed PMID: 35138500]
STEPHENS FD, LENAGHAN D. The anatomical basis and dynamics of vesicoureteral reflux. The Journal of urology. 1962 May:87():669-80 [PubMed PMID: 13916915]
King LR, Kazmi SO, Belman AB. Natural history of vesicoureteral reflux. Outcome of a trial of nonoperative therapy. The Urologic clinics of North America. 1974 Oct:1(3):441-55 [PubMed PMID: 4610948]
Ekman H, Jacobsson B, Kock NG, Sundin T. High diuresis, a factor in preventing vesicoureteral reflux. The Journal of urology. 1966 Apr:95(4):511-5 [PubMed PMID: 5931196]
Kirkland IS, Ross JA, Edmond P, Long WJ. Ureteral function in vesico-ureteral reflux. British journal of urology. 1971 Jun:43(3):289-96 [PubMed PMID: 4933886]
PAQUIN AJ Jr. Ureterovesical anastomosis: the description and evaluation of a technique. The Journal of urology. 1959 Nov:82():573-83 [PubMed PMID: 14430329]
Kalayeh K, Brian Fowlkes J, Schultz WW, Sack BS. The 5:1 rule overestimates the needed tunnel length during ureteral reimplantation. Neurourology and urodynamics. 2021 Jan:40(1):85-94. doi: 10.1002/nau.24526. Epub 2020 Oct 5 [PubMed PMID: 33017072]
Walawender L, Hains DS, Schwaderer AL. Diagnosis and imaging of neonatal UTIs. Pediatrics and neonatology. 2020 Apr:61(2):195-200. doi: 10.1016/j.pedneo.2019.10.003. Epub 2019 Nov 5 [PubMed PMID: 31761714]
Williams G, Hodson EM, Craig JC. Interventions for primary vesicoureteric reflux. The Cochrane database of systematic reviews. 2019 Feb 20:2(2):CD001532. doi: 10.1002/14651858.CD001532.pub5. Epub 2019 Feb 20 [PubMed PMID: 30784039]
Level 1 (high-level) evidenceRadmayr C, Schwentner C, Lunacek A, Karatzas A, Oswald J. Embryology and anatomy of the vesicoureteric junction with special reference to the etiology of vesicoureteral reflux. Therapeutic advances in urology. 2009 Dec:1(5):243-50. doi: 10.1177/1756287209348985. Epub [PubMed PMID: 21789071]
Level 3 (low-level) evidenceRolleston GL, Maling TM, Hodson CJ. Intrarenal reflux and the scarred kidney. Archives of disease in childhood. 1974 Jul:49(7):531-9 [PubMed PMID: 4852544]
Gkalonaki I, Schoina E, Anastasakis M, Patoulias I. Pathogenesis and prognosis of intrarenal reflux. Folia medica Cracoviensia. 2023 Jul 30:63(2):57-64. doi: 10.24425/fmc.2023.145913. Epub [PubMed PMID: 37903379]
Hodson J, Maling TM, McManamon PJ, Lewis MG. Reflux nephropathy. Kidney international. Supplement. 1975 Aug:4():S50-8 [PubMed PMID: 1059812]
Level 3 (low-level) evidenceRisdon RA. The small scarred kidney of childhood. A congenital or an acquired lesion? Pediatric nephrology (Berlin, Germany). 1987 Oct:1(4):632-7 [PubMed PMID: 3153344]
Rose JS, Glassberg KI, Waterhouse K. Intrarenal reflux and its relationship to renal scarring. The Journal of urology. 1975 Mar:113(3):400-3 [PubMed PMID: 1117509]
Ditchfield MR, de Campo JF, Nolan TM, Cook DJ, Grimwood K, Powell HR, Sloane R, Cahill S. Risk factors in the development of early renal cortical defects in children with urinary tract infection. AJR. American journal of roentgenology. 1994 Jun:162(6):1393-7 [PubMed PMID: 8192006]
Level 2 (mid-level) evidenceBenador D, Benador N, Slosman D, Mermillod B, Girardin E. Are younger children at highest risk of renal sequelae after pyelonephritis? Lancet (London, England). 1997 Jan 4:349(9044):17-9 [PubMed PMID: 8988117]
Smellie JM, Ransley PG, Normand IC, Prescod N, Edwards D. Development of new renal scars: a collaborative study. British medical journal (Clinical research ed.). 1985 Jun 29:290(6486):1957-60 [PubMed PMID: 3924325]
McLorie GA, McKenna PH, Jumper BM, Churchill BM, Gilmour RF, Khoury AE. High grade vesicoureteral reflux: analysis of observational therapy. The Journal of urology. 1990 Aug:144(2 Pt 2):537-40; discussion 545 [PubMed PMID: 2374236]
Shimada K, Matsui T, Ogino T, Arima M, Mori Y, Ikoma F. Renal growth and progression of reflux nephropathy in children with vesicoureteral reflux. The Journal of urology. 1988 Nov:140(5 Pt 2):1097-100 [PubMed PMID: 3184282]
Yüksel S. Intrarenal reflux. NDT plus. 2008 Jun:1(3):188-9. doi: 10.1093/ndtplus/sfm050. Epub 2008 Feb 6 [PubMed PMID: 25983875]
Hodson CJ, Maling TM, McManamon PJ, Lewis MG. The pathogenesis of reflux nephropathy (chronic atrophic pyelonephritis). The British journal of radiology. 1975:Suppl 13():1-26 [PubMed PMID: 766885]
Level 3 (low-level) evidenceThomsen HS, Talner LB, Higgins CB. Intrarenal backflow during retrograde pyelography with graded intrapelvic pressure. A radiologic study. Investigative radiology. 1982 Nov-Dec:17(6):593-603 [PubMed PMID: 7152863]
Level 3 (low-level) evidenceTokas T, Herrmann TRW, Skolarikos A, Nagele U, Training and Research in Urological Surgery and Technology (T.R.U.S.T.)-Group. Pressure matters: intrarenal pressures during normal and pathological conditions, and impact of increased values to renal physiology. World journal of urology. 2019 Jan:37(1):125-131. doi: 10.1007/s00345-018-2378-4. Epub 2018 Jun 18 [PubMed PMID: 29915945]
Narchi H, Marah M, Khan AA, Al-Amri A, Al-Shibli A. Renal tract abnormalities missed in a historical cohort of young children with UTI if the NICE and AAP imaging guidelines were applied. Journal of pediatric urology. 2015 Oct:11(5):252.e1-7. doi: 10.1016/j.jpurol.2015.03.010. Epub 2015 Apr 21 [PubMed PMID: 25979215]
Lytzen R, Thorup J, Cortes D. Experience with the NICE guidelines for imaging studies in children with first pyelonephritis. European journal of pediatric surgery : official journal of Austrian Association of Pediatric Surgery ... [et al] = Zeitschrift fur Kinderchirurgie. 2011 Oct:21(5):283-6. doi: 10.1055/s-0031-1277212. Epub 2011 Jun 15 [PubMed PMID: 21678239]
Level 2 (mid-level) evidenceWeiss R, Duckett J, Spitzer A. Results of a randomized clinical trial of medical versus surgical management of infants and children with grades III and IV primary vesicoureteral reflux (United States). The International Reflux Study in Children. The Journal of urology. 1992 Nov:148(5 Pt 2):1667-73 [PubMed PMID: 1433585]
Level 1 (high-level) evidence. Prospective trial of operative versus non-operative treatment of severe vesicoureteric reflux in children: five years' observation. Birmingham Reflux Study Group. British medical journal (Clinical research ed.). 1987 Jul 25:295(6592):237-41 [PubMed PMID: 2888509]
Level 1 (high-level) evidenceLachenmyer LL, Anderson JJ, Clayton DB, Thomas JC, Pope JC 4th, Adams MC, Brock JW 3rd, Tanaka ST. Analysis of an intervention to reduce parental anxiety prior to voiding cystourethrogram. Journal of pediatric urology. 2013 Dec:9(6 Pt B):1223-8. doi: 10.1016/j.jpurol.2013.05.015. Epub 2013 Jun 13 [PubMed PMID: 23769752]
Level 1 (high-level) evidenceHerd DW. Anxiety in children undergoing VCUG: sedation or no sedation? Advances in urology. 2008:2008():498614. doi: 10.1155/2008/498614. Epub [PubMed PMID: 18615194]
Level 3 (low-level) evidenceWu CW, Wei CC, Lin CL, Chao HH, Wei TC, Hsieh TH, Lin CY. Risk factors of vesicoureteral reflux and urinary tract infections in children with imperforate anus: A population-based case-control study in Taiwan. Medicine. 2021 Nov 5:100(44):e27499. doi: 10.1097/MD.0000000000027499. Epub [PubMed PMID: 34871211]
Level 2 (mid-level) evidenceGuerra LA, Keays MA, Purser MJ, Wang SY, Leonard MP. Pediatric cystogram: Are we considering age-adjusted bladder capacity? Canadian Urological Association journal = Journal de l'Association des urologues du Canada. 2018 Dec:12(12):378-381. doi: 10.5489/cuaj.5263. Epub [PubMed PMID: 29940135]
Lendvay TS, Sorensen M, Cowan CA, Joyner BD, Mitchell MM, Grady RW. The evolution of vesicoureteral reflux management in the era of dextranomer/hyaluronic acid copolymer: a pediatric health information system database study. The Journal of urology. 2006 Oct:176(4 Pt 2):1864-7 [PubMed PMID: 16945675]
Dogan HS, Altan M, Citamak B, Bozaci AC, Koni A, Tekgul S. Factors affecting the success of endoscopic treatment of vesicoureteral reflux and comparison of two dextranomer based bulking agents: does bulking substance matter? Journal of pediatric urology. 2015 Apr:11(2):90.e1-5. doi: 10.1016/j.jpurol.2014.12.009. Epub 2015 Mar 2 [PubMed PMID: 25791422]
Level 2 (mid-level) evidenceMoore K, Bolduc S. Prospective study of polydimethylsiloxane vs dextranomer/hyaluronic acid injection for treatment of vesicoureteral reflux. The Journal of urology. 2014 Dec:192(6):1794-9. doi: 10.1016/j.juro.2014.05.116. Epub 2014 Jun 10 [PubMed PMID: 24928269]
Level 1 (high-level) evidenceBabu R, Chandrasekharam VVS. A systematic review & meta-analysis comparing outcomes of endoscopic treatment of primary vesico ureteric reflux in children with polyacrylate poly alcohol copolymer versus dextranomer hyaluranic acid. Journal of pediatric surgery. 2022 Nov:57(11):683-689. doi: 10.1016/j.jpedsurg.2022.01.025. Epub 2022 Feb 1 [PubMed PMID: 35197197]
Level 1 (high-level) evidenceLightfoot M, Bilgutay AN, Tollin N, Eisenberg S, Weiser J, Bryan L, Smith E, Elmore J, Scherz H, Kirsch AJ. Long-Term Clinical Outcomes and Parental Satisfaction After Dextranomer/Hyaluronic Acid (Dx/HA) Injection for Primary Vesicoureteral Reflux. Frontiers in pediatrics. 2019:7():392. doi: 10.3389/fped.2019.00392. Epub 2019 Sep 27 [PubMed PMID: 31612121]
Level 2 (mid-level) evidenceKajbafzadeh AM, Sabetkish S, Khorramirouz R, Sabetkish N. Comparison of histopathological characteristics of polyacrylate polyalcohol copolymer with dextranomer/hyaluronic acid after injection beneath the bladder mucosa layer: a rabbit model. International urology and nephrology. 2017 May:49(5):747-752. doi: 10.1007/s11255-017-1540-z. Epub 2017 Feb 16 [PubMed PMID: 28210914]
Şencan A, Yıldırım H, Özkan KU, Uçan B, Karkıner A, Hoşgör M. Late ureteral obstruction after endoscopic treatment of vesicoureteral reflux with polyacrylate polyalcohol copolymer. Urology. 2014 Nov:84(5):1188-93. doi: 10.1016/j.urology.2014.07.030. Epub 2014 Oct 24 [PubMed PMID: 25443932]
Level 2 (mid-level) evidenceO'Donnell B, Puri P. Treatment of vesicoureteric reflux by endoscopic injection of Teflon. British medical journal (Clinical research ed.). 1984 Jul 7:289(6436):7-9 [PubMed PMID: 6428669]
Capozza N, Caione P. Modification of the sting procedure for vesicoureteral reflux: ureteral repositioning and injection. Archivos espanoles de urologia. 2008 Mar:61(2):254-7 [PubMed PMID: 18491743]
Kaye JD, Srinivasan AK, Delaney C, Cerwinka WH, Elmore JM, Scherz HC, Kirsch AJ. Clinical and radiographic results of endoscopic injection for vesicoureteral reflux: defining measures of success. Journal of pediatric urology. 2012 Jun:8(3):297-303. doi: 10.1016/j.jpurol.2011.02.006. Epub 2011 May 4 [PubMed PMID: 21543259]
Level 2 (mid-level) evidenceYap TL, Chen Y, Nah SA, Ong CC, Jacobsen A, Low Y. STING versus HIT technique of endoscopic treatment for vesicoureteral reflux: A systematic review and meta-analysis. Journal of pediatric surgery. 2016 Dec:51(12):2015-2020. doi: 10.1016/j.jpedsurg.2016.09.028. Epub 2016 Sep 16 [PubMed PMID: 27773360]
Level 1 (high-level) evidenceArlen AM, Kirsch AJ. Predicting Breakthrough Urinary Tract Infection: Comparative Analysis of Vesicoureteral Reflux Index, Reflux Grade and Ureteral Diameter Ratio. Reply. The Journal of urology. 2021 Nov:206(5):1336-1337. doi: 10.1097/JU.0000000000002166. Epub 2021 Aug 12 [PubMed PMID: 34383601]
Level 2 (mid-level) evidenceKirsch AJ, Arlen AM, Lackgren G. Current trends in dextranomer hyaluronic acid copolymer (Deflux) injection technique for endoscopic treatment of vesicoureteral reflux. Urology. 2014 Aug:84(2):462-8. doi: 10.1016/j.urology.2014.04.032. Epub 2014 Jun 26 [PubMed PMID: 24975706]
Law ZW, Ong CCP, Yap TL, Loh AHP, Joseph U, Sim SW, Ong LY, Low Y, Jacobsen AS, Chen Y. Extravesical vs. intravesical ureteric reimplantation for primary vesicoureteral reflux: A systematic review and meta-analysis. Frontiers in pediatrics. 2022:10():935082. doi: 10.3389/fped.2022.935082. Epub 2022 Oct 21 [PubMed PMID: 36340705]
Level 1 (high-level) evidencePuri P, Kutasy B, Colhoun E, Hunziker M. Single center experience with endoscopic subureteral dextranomer/hyaluronic acid injection as first line treatment in 1,551 children with intermediate and high grade vesicoureteral reflux. The Journal of urology. 2012 Oct:188(4 Suppl):1485-9. doi: 10.1016/j.juro.2012.02.023. Epub 2012 Aug 17 [PubMed PMID: 22906657]
Level 2 (mid-level) evidenceStenbäck A, Olafsdottir T, Sköldenberg E, Barker G, Stenberg A, Läckgren G. Proprietary non-animal stabilized hyaluronic acid/dextranomer gel (NASHA/Dx) for endoscopic treatment of grade IV vesicoureteral reflux: Long-term observational study. Journal of pediatric urology. 2020 Jun:16(3):328.e1-328.e9. doi: 10.1016/j.jpurol.2020.04.008. Epub 2020 Apr 13 [PubMed PMID: 32414615]
Level 2 (mid-level) evidenceKirsch AJ, Perez-Brayfield M, Smith EA, Scherz HC. The modified sting procedure to correct vesicoureteral reflux: improved results with submucosal implantation within the intramural ureter. The Journal of urology. 2004 Jun:171(6 Pt 1):2413-6 [PubMed PMID: 15126864]
Routh JC, Inman BA, Reinberg Y. Dextranomer/hyaluronic acid for pediatric vesicoureteral reflux: systematic review. Pediatrics. 2010 May:125(5):1010-9. doi: 10.1542/peds.2009-2225. Epub 2010 Apr 5 [PubMed PMID: 20368325]
Level 1 (high-level) evidenceGupta A, Snodgrass W. Intra-orifice versus hydrodistention implantation technique in dextranomer/hyaluronic acid injection for vesicoureteral reflux. The Journal of urology. 2008 Oct:180(4 Suppl):1589-92; discussion 1592-3. doi: 10.1016/j.juro.2008.04.073. Epub 2008 Aug 16 [PubMed PMID: 18710753]
Yucel S, Gupta A, Snodgrass W. Multivariate analysis of factors predicting success with dextranomer/hyaluronic acid injection for vesicoureteral reflux. The Journal of urology. 2007 Apr:177(4):1505-9 [PubMed PMID: 17382765]
Kirsch AJ, Perez-Brayfield MR, Scherz HC. Minimally invasive treatment of vesicoureteral reflux with endoscopic injection of dextranomer/hyaluronic acid copolymer: the Children's Hospitals of Atlanta experience. The Journal of urology. 2003 Jul:170(1):211-5 [PubMed PMID: 12796692]
Dalkiliç A, Bayar G, Demirkan H, Horasanli K. The learning curve of sting method for endoscopic injection treatment of vesicoureteral reflux. International braz j urol : official journal of the Brazilian Society of Urology. 2018 Nov-Dec:44(6):1200-1206. doi: 10.1590/S1677-5538.IBJU.2017.0465. Epub [PubMed PMID: 30325598]
Lorenzo AJ, Pippi Salle JL, Barroso U, Cook A, Grober E, Wallis MC, Bägli DJ, Khoury AE. What are the most powerful determinants of endoscopic vesicoureteral reflux correction? Multivariate analysis of a single institution experience during 6 years. The Journal of urology. 2006 Oct:176(4 Pt 2):1851-5 [PubMed PMID: 16945671]
Dave S, Bägli DJ. A review of the effect of injected dextranomer/hyaluronic Acid copolymer volume on reflux correction following endoscopic injection. Advances in urology. 2008:2008():579370. doi: 10.1155/2008/579370. Epub [PubMed PMID: 18827895]
Level 3 (low-level) evidenceHidas G, Soltani T, Watts B, Pribish M, Khoury AE. Is the appearance of the dextranomer/hyaluronic acid mound predictive of reflux resolution? The Journal of urology. 2013 May:189(5):1882-5. doi: 10.1016/j.juro.2012.11.110. Epub 2012 Nov 27 [PubMed PMID: 23201379]
Dwyer ME, Husmann DA, Rathbun SR, Weight CJ, Kramer SA. Febrile urinary tract infections after ureteroneocystostomy and subureteral injection of dextranomer/hyaluronic acid for vesicoureteral reflux--do choice of procedure and success matter? The Journal of urology. 2013 Jan:189(1):275-82. doi: 10.1016/j.juro.2012.09.011. Epub 2012 Nov 20 [PubMed PMID: 23174239]
Level 2 (mid-level) evidenceKraft KH, Molitierno JA Jr, Dewhurst L, Geers C, Gunderson K, Scherz HC, Kirsch AJ. Is endoscopic injection therapy a reasonable treatment option for low-grade vesicoureteral reflux in association with overactive bladder? Urology. 2011 Sep:78(3):675-8. doi: 10.1016/j.urology.2010.12.084. Epub 2011 May 7 [PubMed PMID: 21550643]
Cocomazzi R, Salatto A, Campanella V, Pastore V, Maggipinto C, Aceto G, Bartoli F. Bladder Dysfunction and Re-Absorbable Bulking Agent Affect Success Rate in Children Underwent Endoscopic Treatment for Vesicoureteral Reflux: A Long-Term Follow-Up Study. Children (Basel, Switzerland). 2021 Oct 1:8(10):. doi: 10.3390/children8100875. Epub 2021 Oct 1 [PubMed PMID: 34682140]
Van Batavia JP, Nees SN, Fast AM, Combs AJ, Glassberg KI. Outcomes of vesicoureteral reflux in children with non-neurogenic lower urinary tract dysfunction treated with dextranomer/hyaluronic acid copolymer (Deflux). Journal of pediatric urology. 2014 Jun:10(3):482-7. doi: 10.1016/j.jpurol.2013.10.017. Epub 2013 Nov 12 [PubMed PMID: 24290224]
Level 2 (mid-level) evidenceFriedmacher F, Colhoun E, Puri P. Endoscopic Injection of Dextranomer/Hyaluronic Acid as First Line Treatment in 851 Consecutive Children with High Grade Vesicoureteral Reflux: Efficacy and Long-Term Results. The Journal of urology. 2018 Sep:200(3):650-655. doi: 10.1016/j.juro.2018.03.074. Epub 2018 Mar 15 [PubMed PMID: 29551405]
Kalisvaart JF, Scherz HC, Cuda S, Kaye JD, Kirsch AJ. Intermediate to long-term follow-up indicates low risk of recurrence after Double HIT endoscopic treatment for primary vesico-ureteral reflux. Journal of pediatric urology. 2012 Aug:8(4):359-65. doi: 10.1016/j.jpurol.2011.07.006. Epub 2011 Aug 4 [PubMed PMID: 21820358]
Level 2 (mid-level) evidenceElder JS, Diaz M, Caldamone AA, Cendron M, Greenfield S, Hurwitz R, Kirsch A, Koyle MA, Pope J, Shapiro E. Endoscopic therapy for vesicoureteral reflux: a meta-analysis. I. Reflux resolution and urinary tract infection. The Journal of urology. 2006 Feb:175(2):716-22 [PubMed PMID: 16407037]
Level 1 (high-level) evidenceHUTCH JA. Vesico-ureteral reflux in the paraplegic: cause and correction. The Journal of urology. 1952 Aug:68(2):457-69 [PubMed PMID: 14955874]
Politano VA, Leadbetter WF. An Operative Technique for the Correction of Vesicoureteral Reflux. The Journal of urology. 2017 Feb:197(2S):S94-S100. doi: 10.1016/j.juro.2016.10.093. Epub 2016 Dec 21 [PubMed PMID: 28012771]
Glenn JF, Anderson EE. Distal tunnel ureteral reimplantation. The Journal of urology. 1967 Apr:97(4):623-6 [PubMed PMID: 6022427]
POLITANO VA, LEADBETTER WF. An operative technique for the correction of vesicoureteral reflux. The Journal of urology. 1958 Jun:79(6):932-41 [PubMed PMID: 13539988]
Cohen SJ. The Cohen reimplantation technique. Birth defects original article series. 1977:13(5):391-5 [PubMed PMID: 588711]
Ozdemir T, Sayan A, Koyluoglu G. Modified Leadbetter-Politano Ureteroneocystostomy: A Safer Procedure. Urology journal. 2020 Aug 23:17(5):501-504. doi: 10.22037/uj.v16i7.5709. Epub 2020 Aug 23 [PubMed PMID: 32869253]
Passoni N, Peters CA. Robotic Ureteral Reimplantation. Journal of endourology. 2020 May:34(S1):S31-S34. doi: 10.1089/end.2019.0619. Epub 2020 Mar 24 [PubMed PMID: 32207993]
Steffens J, Stark E, Haben B, Treiyer A. Politano-Leadbetter ureteric reimplantation. BJU international. 2006 Sep:98(3):695-712 [PubMed PMID: 16925783]
Soh S, Kobori Y, Shin T, Suzuki K, Iwahata T, Sadaoka Y, Sato R, Nishi M, Iwamura M, Okada H. Transvesicoscopic ureteral reimplantation: Politano-Leadbetter versus Cohen technique. International journal of urology : official journal of the Japanese Urological Association. 2015 Apr:22(4):394-9. doi: 10.1111/iju.12702. Epub 2015 Mar 9 [PubMed PMID: 25754455]
Level 2 (mid-level) evidenceGlenn JF, Anderson EE. Distal tunnel ureteral reimplantation. Transactions of the American Association of Genito-Urinary Surgeons. 1966:58():37-41 [PubMed PMID: 5963385]
Nishi M, Eura R, Hayashi C, Gohbara A, Yamazaki Y. Vesicoscopic ureteral reimplantation with a modified Glenn-Anderson technique for vesicoureteral reflux. Journal of pediatric urology. 2023 Jun:19(3):322.e1-322.e7. doi: 10.1016/j.jpurol.2023.02.018. Epub 2023 Feb 26 [PubMed PMID: 36959038]
Palmer JS. Extravesical ureteral reimplantation: an outpatient procedure. The Journal of urology. 2008 Oct:180(4 Suppl):1828-31; discussion 1831. doi: 10.1016/j.juro.2008.04.080. Epub 2008 Aug 21 [PubMed PMID: 18721936]
Riedmiller H, Gerharz EW. Antireflux surgery: Lich-Gregoir extravesical ureteric tunnelling. BJU international. 2008 Jun:101(11):1467-82. doi: 10.1111/j.1464-410X.2008.07683.x. Epub [PubMed PMID: 18454801]
GREGOIR W. [THE SURGICAL TREATMENT OF CONGENITAL VESICO-URETERAL REFLUX]. Acta chirurgica Belgica. 1964 Apr:63():431-9 [PubMed PMID: 14197630]
LICH R Jr, HOWERTON LW, DAVIS LA. Ureteral reflux, its significance and correction. Southern medical journal. 1962 Jun:55():633-5 [PubMed PMID: 14465077]
Lee RS, Bakthavatsalam R, Marsh CL, Kuhr CS. Ureteral complications in renal transplantation: a comparison of the Lich-Gregoir versus the Taguchi technique. Transplantation proceedings. 2007 Jun:39(5):1461-4 [PubMed PMID: 17580162]
Silay MS, Turan T, Kayalı Y, Başıbüyük İ, Gunaydin B, Caskurlu T, Karaman Mİ. Comparison of intravesical (Cohen) and extravesical (Lich-Gregoir) ureteroneocystostomy in the treatment of unilateral primary vesicoureteric reflux in children. Journal of pediatric urology. 2018 Feb:14(1):65.e1-65.e4. doi: 10.1016/j.jpurol.2017.09.014. Epub 2017 Oct 14 [PubMed PMID: 29146303]
Kruppa C, Fitze G, Schuchardt K. Vesicoscopic Cross-Trigonal Ureteral Reimplantation for Vesicoureteral Reflux: Intermediate Results. Children (Basel, Switzerland). 2022 Feb 21:9(2):. doi: 10.3390/children9020298. Epub 2022 Feb 21 [PubMed PMID: 35205018]
Canon SJ, Jayanthi VR, Patel AS. Vesicoscopic cross-trigonal ureteral reimplantation: a minimally invasive option for repair of vesicoureteral reflux. The Journal of urology. 2007 Jul:178(1):269-73; discussion 273 [PubMed PMID: 17499791]
Li W, Dong H, Chen P, Su C, Wang C, Li Y, Li Y, Chen J, Luo Y. Surgical Management of Vesicoureteral Junction Obstruction in Children: A Comparative Study Between Transvesicoscopic Cohen Reimplantation and Transumbilical Laparoendoscopic Single-Site Lich-Gregoir Techniques. Journal of endourology. 2022 Aug:36(8):1043-1049. doi: 10.1089/end.2021.0309. Epub 2022 May 2 [PubMed PMID: 35323047]
Level 2 (mid-level) evidenceDavid S, Kelly C, Poppas DP. Nerve sparing extravesical repair of bilateral vesicoureteral reflux: description of technique and evaluation of urinary retention. The Journal of urology. 2004 Oct:172(4 Pt 2):1617-20; discussion 1620 [PubMed PMID: 15371774]
Level 2 (mid-level) evidenceSoulier V, Scalabre AL, Lopez M, Li CY, Thach S, Vermersch S, Varlet FO. Laparoscopic vesico-ureteral reimplantation with Lich-Gregoir approach in children: medium term results of 159 renal units in 117 children. World journal of urology. 2017 Nov:35(11):1791-1798. doi: 10.1007/s00345-017-2064-y. Epub 2017 Jun 21 [PubMed PMID: 28638940]
Traxel EJ, Minevich EA, Noh PH. A review: the application of minimally invasive surgery to pediatric urology: lower urinary tract reconstructive procedures. Urology. 2010 Jul:76(1):115-20. doi: 10.1016/j.urology.2009.11.073. Epub 2010 Mar 29 [PubMed PMID: 20303150]
Szavay PO. Applications of Laparoscopic Transperitoneal Surgery of the Pediatric Urinary Tract. Frontiers in pediatrics. 2019:7():29. doi: 10.3389/fped.2019.00029. Epub 2019 Feb 11 [PubMed PMID: 30805327]
Herz D, Fuchs M, Todd A, McLeod D, Smith J. Robot-assisted laparoscopic extravesical ureteral reimplant: A critical look at surgical outcomes. Journal of pediatric urology. 2016 Dec:12(6):402.e1-402.e9. doi: 10.1016/j.jpurol.2016.05.042. Epub 2016 Jul 26 [PubMed PMID: 27522319]
Zaontz MR, Maizels M, Sugar EC, Firlit CF. Detrusorrhaphy: extravesical ureteral advancement to correct vesicoureteral reflux in children. The Journal of urology. 1987 Oct:138(4 Pt 2):947-9 [PubMed PMID: 3656576]
Level 2 (mid-level) evidenceWacksman J, Gilbert A, Sheldon CA. Results of the renewed extravesical reimplant for surgical correction of vesicoureteral reflux. The Journal of urology. 1992 Aug:148(2 Pt 1):359-61 [PubMed PMID: 1635135]
Kurtz MP, Leow JJ, Varda BK, Logvinenko T, Yu RN, Nelson CP, Chung BI, Chang SL. Robotic versus open pediatric ureteral reimplantation: Costs and complications from a nationwide sample. Journal of pediatric urology. 2016 Dec:12(6):408.e1-408.e6. doi: 10.1016/j.jpurol.2016.06.016. Epub 2016 Aug 23 [PubMed PMID: 27593917]
Wang HH, Tejwani R, Cannon GM Jr, Gargollo PC, Wiener JS, Routh JC. Open versus minimally invasive ureteroneocystostomy: A population-level analysis. Journal of pediatric urology. 2016 Aug:12(4):232.e1-6. doi: 10.1016/j.jpurol.2016.03.014. Epub 2016 Apr 16 [PubMed PMID: 27140001]
Marchini GS, Hong YK, Minnillo BJ, Diamond DA, Houck CS, Meier PM, Passerotti CC, Kaplan JR, Retik AB, Nguyen HT. Robotic assisted laparoscopic ureteral reimplantation in children: case matched comparative study with open surgical approach. The Journal of urology. 2011 May:185(5):1870-5. doi: 10.1016/j.juro.2010.12.069. Epub 2011 Mar 21 [PubMed PMID: 21421223]
Level 2 (mid-level) evidenceGrimsby GM, Dwyer ME, Jacobs MA, Ost MC, Schneck FX, Cannon GM, Gargollo PC. Multi-institutional review of outcomes of robot-assisted laparoscopic extravesical ureteral reimplantation. The Journal of urology. 2015 May:193(5 Suppl):1791-5. doi: 10.1016/j.juro.2014.07.128. Epub 2014 Oct 7 [PubMed PMID: 25301094]
Boysen WR, Ellison JS, Kim C, Koh CJ, Noh P, Whittam B, Palmer B, Shukla A, Kirsch A, Gundeti MS. Multi-Institutional Review of Outcomes and Complications of Robot-Assisted Laparoscopic Extravesical Ureteral Reimplantation for Treatment of Primary Vesicoureteral Reflux in Children. The Journal of urology. 2017 Jun:197(6):1555-1561. doi: 10.1016/j.juro.2017.01.062. Epub 2017 Jan 24 [PubMed PMID: 28130103]
Chandrasekharam VVS, Babu R. Robot-assisted laparoscopic extravesical versus conventional laparoscopic extravesical ureteric reimplantation for pediatric primary vesicoureteric reflux: a systematic review and meta-analysis. Pediatric surgery international. 2020 Nov:36(11):1371-1378. doi: 10.1007/s00383-020-04749-2. Epub 2020 Sep 27 [PubMed PMID: 32980963]
Level 1 (high-level) evidenceTessier B, Scalabre A, Harper L, Garnier S, Vermesch S, Lopez C, Cazals A, Fila M, Morin D, Varlet F, Kalfa N. Comparative study of open, laparoscopic and endoscopic treatments of intermediate grade vesicoureteral reflux in children. Surgical endoscopy. 2023 Apr:37(4):2682-2687. doi: 10.1007/s00464-021-08985-y. Epub 2022 Nov 22 [PubMed PMID: 36414870]
Level 2 (mid-level) evidenceHubert KC, Kokorowski PJ, Huang L, Prasad MM, Rosoklija I, Retik AB, Nelson CP. Clinical outcomes and long-term resolution in patients with persistent vesicoureteral reflux after open ureteral reimplantation. The Journal of urology. 2012 Oct:188(4 Suppl):1474-9. doi: 10.1016/j.juro.2012.03.048. Epub 2012 Aug 17 [PubMed PMID: 22906647]
Level 2 (mid-level) evidenceKruppa C, Wilke A, Hörz C, Kosk T, Hörz T, Fitze G, Schuchardt K. Vesicoscopic vs. Open Ureteral Reimplantation According to Cohen and Leadbetter-Politano for Vesicoureteral Reflux. Journal of clinical medicine. 2023 Aug 31:12(17):. doi: 10.3390/jcm12175686. Epub 2023 Aug 31 [PubMed PMID: 37685751]
Yağız B, Demirel BD. Ureteral reimplantation aligned laparoscopically: Pneumovesicoscopic Politano-Leadbetter reimplantation in children. Journal of pediatric urology. 2021 Jun:17(3):413.e1-413.e8. doi: 10.1016/j.jpurol.2021.02.007. Epub 2021 Feb 16 [PubMed PMID: 33637456]
Perisinakis K, Raissaki M, Damilakis J, Stratakis J, Neratzoulakis J, Gourtsoyiannis N. Fluoroscopy-controlled voiding cystourethrography in infants and children: are the radiation risks trivial? European radiology. 2006 Apr:16(4):846-51 [PubMed PMID: 16328446]
Tzanis E, Raissaki M, Konstantinos A, Damilakis J, Perisinakis K. Radiation exposure to infants undergoing voiding cystourethrography: The importance of the digital imaging technology. Physica medica : PM : an international journal devoted to the applications of physics to medicine and biology : official journal of the Italian Association of Biomedical Physics (AIFB). 2021 May:85():123-128. doi: 10.1016/j.ejmp.2021.05.006. Epub 2021 May 14 [PubMed PMID: 34000681]
Linke SY, Tsiflikas I, Herz K, Szavay P, Gatidis S, Schäfer JF. Ultra low-dose VCUG in children using a modern flat detectorunit. European radiology. 2016 Jun:26(6):1678-85. doi: 10.1007/s00330-015-3996-5. Epub 2015 Sep 18 [PubMed PMID: 26385801]
Arbique GM, Gilleran JP, Guild JB, Harris JE, Poon CI, Zimmern PE. Radiation exposure during standing voiding cystourethrography in women. Urology. 2006 Feb:67(2):269-74 [PubMed PMID: 16461076]
Rachmiel M, Aladjem M, Starinsky R, Strauss S, Villa Y, Goldman M. Symptomatic urinary tract infections following voiding cystourethrography. Pediatric nephrology (Berlin, Germany). 2005 Oct:20(10):1449-52 [PubMed PMID: 16047224]
Level 2 (mid-level) evidenceUhari M, Nuutinen M, Turtinen J. Adverse reactions in children during long-term antimicrobial therapy. The Pediatric infectious disease journal. 1996 May:15(5):404-8 [PubMed PMID: 8724061]
Mikkelsen LF, Rubak S. Reversible lung fibrosis in a 6-year-old girl after long term nitrofurantoin treatment. BMC pulmonary medicine. 2020 Nov 26:20(1):313. doi: 10.1186/s12890-020-01353-x. Epub 2020 Nov 26 [PubMed PMID: 33243181]
Hunziker M, Mohanan N, D'Asta F, Puri P. Incidence of febrile urinary tract infections in children after successful endoscopic treatment of vesicoureteral reflux: a long-term follow-up. The Journal of pediatrics. 2012 Jun:160(6):1015-20. doi: 10.1016/j.jpeds.2011.12.033. Epub 2012 Jan 28 [PubMed PMID: 22284917]
Level 2 (mid-level) evidenceMinevich E, Wacksman J, Lewis AG, Sheldon CA. Incidence of contralateral vesicoureteral reflux following unilateral extravesical detrusorrhaphy (ureteroneocystostomy). The Journal of urology. 1998 Jun:159(6):2126-8 [PubMed PMID: 9598556]
Level 2 (mid-level) evidenceKoyle MA, Elder JS, Skoog SJ, Mattoo TK, Pohl HG, Reddy PP, Abidari JM, Snodgrass WT. Febrile urinary tract infection, vesicoureteral reflux, and renal scarring: current controversies in approach to evaluation. Pediatric surgery international. 2011 Apr:27(4):337-46. doi: 10.1007/s00383-011-2863-y. Epub 2011 Feb 9 [PubMed PMID: 21305381]
Ransley PG, Risdon RA, Godley ML. High pressure sterile vesicoureteral reflux and renal scarring: an experimental study in the pig and minipig. Contributions to nephrology. 1984:39():320-43 [PubMed PMID: 6086232]
Level 3 (low-level) evidenceSpencer JD, Bates CM, Mahan JD, Niland ML, Staker SR, Hains DS, Schwaderer AL. The accuracy and health risks of a voiding cystourethrogram after a febrile urinary tract infection. Journal of pediatric urology. 2012 Feb:8(1):72-6. doi: 10.1016/j.jpurol.2010.10.012. Epub 2010 Dec 3 [PubMed PMID: 21126919]
Level 2 (mid-level) evidenceKim SW, Im YJ, Hong CH, Han SW. The Clinical Significance of Intrarenal Reflux in Voiding Cystourethrography (VCUG). Korean journal of urology. 2010 Jan:51(1):60-3. doi: 10.4111/kju.2010.51.1.60. Epub 2010 Jan 21 [PubMed PMID: 20414413]
Haid B, Becker T, Koen M, Berger C, Langsteger W, Gruy B, Putz E, Haid S, Oswald J. Lower radiation burden in state of the art fluoroscopic cystography compared to direct isotope cystography in children. Journal of pediatric urology. 2015 Feb:11(1):35.e1-6. doi: 10.1016/j.jpurol.2014.08.015. Epub 2015 Feb 7 [PubMed PMID: 25748630]
Level 2 (mid-level) evidenceMane N, Sharma A, Patil A, Gadekar C, Andankar M, Pathak H. Comparison of contrast-enhanced voiding urosonography with voiding cystourethrography in pediatric vesicoureteral reflux. Turkish journal of urology. 2018 May:44(3):261-267. doi: 10.5152/tud.2018.76702. Epub 2018 Mar 6 [PubMed PMID: 29733800]
Cooper CS, Birusingh KK, Austin JC, Knudson MJ, Brophy PD. Distal ureteral diameter measurement objectively predicts vesicoureteral reflux outcome. Journal of pediatric urology. 2013 Feb:9(1):99-103. doi: 10.1016/j.jpurol.2011.12.011. Epub 2012 Jan 9 [PubMed PMID: 22236467]
Level 2 (mid-level) evidenceArlen AM. Editorial Comment. The Journal of urology. 2021 Sep:206(3):743. doi: 10.1097/JU.0000000000001869.01. Epub 2021 Jun 21 [PubMed PMID: 34148368]
Level 3 (low-level) evidenceWildbrett P, Schwebs M, Abel JR, Lode H, Barthlen W. Spontaneous vesicoureteral reflux resolution in children: A ten-year single-centre experience. African journal of paediatric surgery : AJPS. 2013 Jan-Apr:10(1):9-12. doi: 10.4103/0189-6725.109375. Epub [PubMed PMID: 23519850]
Level 2 (mid-level) evidencede Bessa J Jr, de Carvalho Mrad FC, Mendes EF, Bessa MC, Paschoalin VP, Tiraboschi RB, Sammour ZM, Gomes CM, Braga LH, Bastos Netto JM. Antibiotic prophylaxis for prevention of febrile urinary tract infections in children with vesicoureteral reflux: a meta-analysis of randomized, controlled trials comparing dilated to nondilated vesicoureteral reflux. The Journal of urology. 2015 May:193(5 Suppl):1772-7. doi: 10.1016/j.juro.2014.10.092. Epub 2015 Mar 25 [PubMed PMID: 25817142]
Level 1 (high-level) evidenceHajiyev P, Burgu B. Contemporary Management of Vesicoureteral Reflux. European urology focus. 2017 Apr:3(2-3):181-188. doi: 10.1016/j.euf.2017.08.012. Epub 2017 Sep 12 [PubMed PMID: 28918954]
Sung J, Skoog S. Surgical management of vesicoureteral reflux in children. Pediatric nephrology (Berlin, Germany). 2012 Apr:27(4):551-61. doi: 10.1007/s00467-011-1933-7. Epub 2011 Jun 22 [PubMed PMID: 21695451]
Salmon K, McGuigan F, Pereira JK. Brief report: Optimizing children's memory and management of an invasive medical procedure: the influence of procedural narration and distraction. Journal of pediatric psychology. 2006 Jun:31(5):522-7 [PubMed PMID: 16177227]
Hernanz-Schulman M, Goske MJ, Bercha IH, Strauss KJ. Pause and pulse: ten steps that help manage radiation dose during pediatric fluoroscopy. AJR. American journal of roentgenology. 2011 Aug:197(2):475-81. doi: 10.2214/AJR.10.6122. Epub [PubMed PMID: 21785097]