Definition/Introduction
Stereognosis is the ability to identify the shape and form of a three-dimensional object and, therefore, its identity with tactile manipulation of that object in the absence of visual and auditory stimuli. The etymology of the word stereognosis is from the Greek for "stereo," meaning solid, and "gnosis," meaning knowledge. A distinction is necessary between manual and oral stereognosis, which occurs in the hands and mouth.[1]
Manual stereognosis requires intact peripheral sensory pathways, namely the dorsal column-medial lemniscus tract (DCMLT), to receive discriminative touch and proprioceptive information. Receival of this information is necessary but not sufficient for stereognosis because it also requires functioning processing centers in the cortex of the parietal lobe. See Image. The Sensory Tract.
To properly understand the clinical relevance of stereognosis, one must first appreciate the primary pathway that allows perception of the size and shape of the object, the DCMLT, and how this information is processed in the parietal cortex.
The first-order neurons of the DCMLT have cell bodies in dorsal root ganglia with peripheral processes extending out to sensory receptors (Pacinian corpuscles, Merkel cells, Golgi tendon organs, and muscle spindles) and central processes that enter the spinal cord in the large sensory fiber entry zone of the dorsal horn ascending ipsilaterally in the dorsal columns.[2]
Nerve fibers introduced to the dorsal columns from the lower extremities localize to the medial aspect as they ascend ipsilaterally, forming bundles of axons called the fasciculi gracilis. Similar proprioceptive and discriminative touch information from the upper extremities (spinal level T5 and above) localizes to adjacent lateral axon bundles called the fasciculi cuneatus. The fact that nerve fibers enter the spinal cord and add to the dorsal column fascicles in a medial-to-lateral manner is an important concept. This somatotopic organization of the ascending tracts allows for precise localization of these stimuli when they eventually reach the primary somatosensory cortex of the parietal lobe.[3]
However, before this can occur, the fasciculi cuneatus and gracilis must ascend ipsilaterally until they reach the caudal medulla. As these axon bundles enter the caudal medulla, the neurons synapse in their respective nuclei: the nuclei cuneatus and nuclei gracilis. Second-order neurons of the DCMLT decussate, or cross over, in the caudal medulla, forming crossing tracts termed the internal arcuate fibers. This important anatomic process explains why the interpretation of right-sided discriminative touch information is via the left somatosensory cortex and vice versa. After decussation, the internal arcuate fibers are now referred to as the medial lemnisci and travel through the pons and midbrain until reaching the thalami of the diencephalon. Here, second-order neurons synapse with third-order neurons in the ventral posterolateral nuclei of the thalami. Third-order neurons from each tract pass through their respective internal capsule en route to the primary somatosensory cortices of the anterior parietal lobes located in the postcentral gyri.[2][4][5]
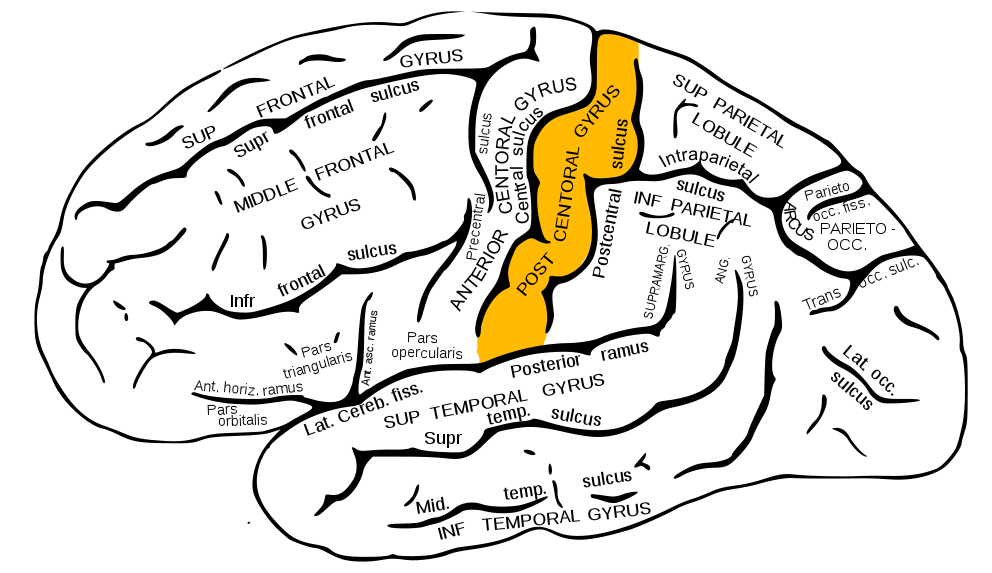
The organization of the parietal lobes' primary somatosensory cortices is somatotopic such that the inferior anatomic structure information (from the lower extremities) is interpreted in the superomedial aspect of the postcentral gyrus (see Image. Postcentral Gyrus of Anterior Parietal Lobe). The superior anatomic structure information (from the face and arms) is interpreted in the lateral aspect of said gyrus. This organization's representation is as a diagrammatic homunculus overlying the precentral gyrus, with areas of the body being displayed in their appropriate location along the gyrus and the anatomic structure size being proportional to their relative tactile sensitivity. During manual stereognosis, information from the hand portion of the primary somatosensory cortex is sent to the tertiary somatosensory association cortex located in the superior parietal lobule. Localization of stimuli and information integration within this cortical processing area represent the physiologic basis for stereognosis.[6] See Image. Sensory Homunculus.
Issues of Concern
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Issues of Concern
Terminology Confusion
It is crucial to understand stereognosis relative to a set of related terms: astereognosis, agnosia, and tactile object agnosia. Recall that stereognosis is the ability to perceive an object's physical form and identity based on tactile stimuli alone. Astereognosis refers to the inability to perceive the form and identity of an object when physically manipulating it through active touch. By definition, astereognosis necessitates functioning peripheral sensory modalities (pain, temperature, fine touch, and vibration sense) and results from pathology within the central cortical integration of this sensory information. Astereognosis falls under a family of conditions called agnosias, which are classified based on the sensory modality involved (auditory, visual, or tactile). In this terminology, one should also be aware that tactile object agnosia is used interchangeably and practically synonymous with astereognosis.[7][8]
Limitations of Testing
Because stereognosis is a function of cortical sensory areas, it is worth noting that cortical sensory function is not testable if the patient has peripheral lesions that cause dysfunction in the afferent pathways that supply the cerebrum. For example, suppose a patient has peripheral neuropathy that limits the ability for tactile signals to reach the brain. In that case, it can be challenging to ascertain whether or not the patient's stereognosis limitations are secondary to their peripheral neuropathy or a cortical processing deficit.[9]
Clinical Significance
Clinical Testing
It is important to understand stereognosis assessments in general sensory function due to the dependent relationship between cortical sensory processing and afferent sensation. Tests of peripheral sensation often include sensation to light touch (using fingertip palpation or cotton swab), pain (alternating between relatively sharp and dull stimuli), temperature (often using a cool metallic object), vibration (using a tuning fork), and two-point discrimination (using calipers). If all these functions are intact, the patient's cortical sensory function can be assessed. To test stereognosis specifically, clinicians often perform a tactile object recognition (TOR) test. The TOR test asks the patient to close their eyes, place a series of common objects in the patient's hand, and ask them to identify the object. The objects frequently used in the TOR test include a pen, key, comb, and paperclip. If the patient can recognize the object in their hand, their stereognosis is considered intact. They are said to have astereognosis if they cannot identify the object despite having an intact peripheral sensory function.[9][10]
Astereognosis
Astereognosis indicates that a patient suffers from a lesion of the primary somatosensory cortex or somatosensory association area of the parietal lobe, commonly described as a cortical sensory loss. As previously discussed, afferent sensory fibers located in the periphery decussate before reaching the cerebral cortex, which means that defects in these cortical processing regions produce contralateral deficits. Fine motor functions involved with activities of daily living, such as feeding oneself, require coordination between sensory and motor processing areas of the cortex. Therefore, defects in the processing of haptic information can result in difficulty performing activities of daily living and impede the recovery of patients with cortical injuries. Many conditions precipitate astereognosis, including but not limited to cortical dementias, cerebral palsy, cerebrovascular stroke, and meningioma.[11]
Cerebrovascular Strokes
Between 2013 and 2016, approximately 7 million Americans reported having a stroke, with a significant proportion of these individuals (17%) being above the age of 85. It is widely recognized that the motor and sensory deficits following strokes, including cortical sensory modalities like stereognosis, represent a large portion of the burden of disease and recovery. With the high prevalence of cerebrovascular strokes, there is a great opportunity for improving outcomes and recovery. This is of particular interest in the elderly populations who tend to have poorer outcomes. In cases of cortical sensorimotor stroke, tracking recovery and rehabilitation status often includes an assessment of tactile object recognition. Also, many of the therapeutic modalities that allow stroke patients to regain their functional status focus on sensory discrimination and recognition skills. Thus, stereognosis provides great clinical utility in prognostication and maximizing sensorimotor recovery following cerebrovascular strokes.[12][13][14][15][16]
Nursing, Allied Health, and Interprofessional Team Interventions
Post-Stroke Sensory Retraining
Studies have suggested a degree of sensorimotor neuronal recovery following strokes, particularly for proprioception and stereognosis. It has also been recognized that sensorimotor deficits contribute to interference with activities of daily living and return to functional status in patients recovering from a stroke. That said, there is significant variability in the standardized outcomes used by physicians, physical therapists, and occupational therapists to monitor stroke patient recovery status. Also, the rehabilitation community lacks consensus about the physical therapy protocol that optimizes sensorimotor recovery. The American Stroke Association guidelines indicate that the use of somatosensory retraining to improve sensory discrimination can be an option for stroke survivors who demonstrate sensory deficits. For these reasons, it may benefit patients if physicians and physical/occupational therapy staff coordinate to construct a regimen that includes sensory retraining to improve the performance of activities of daily living in stroke survivors.[12][17][18][19]
Media
(Click Image to Enlarge)
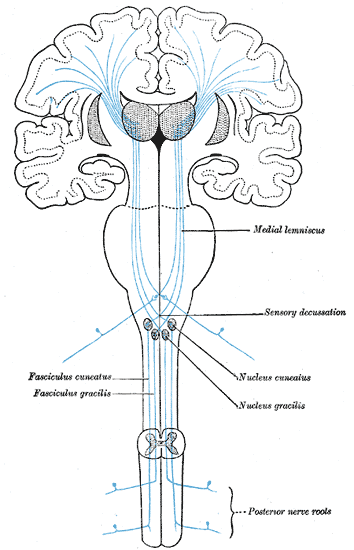
The Sensory Tract. The illustration depicts the medial lemniscus, fasciculus cuneatus, fasciculus gracilis, sensory decussation, nucleus cuneatus, nucleus gracilis, and posterior nerve roots.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
(Click Image to Enlarge)
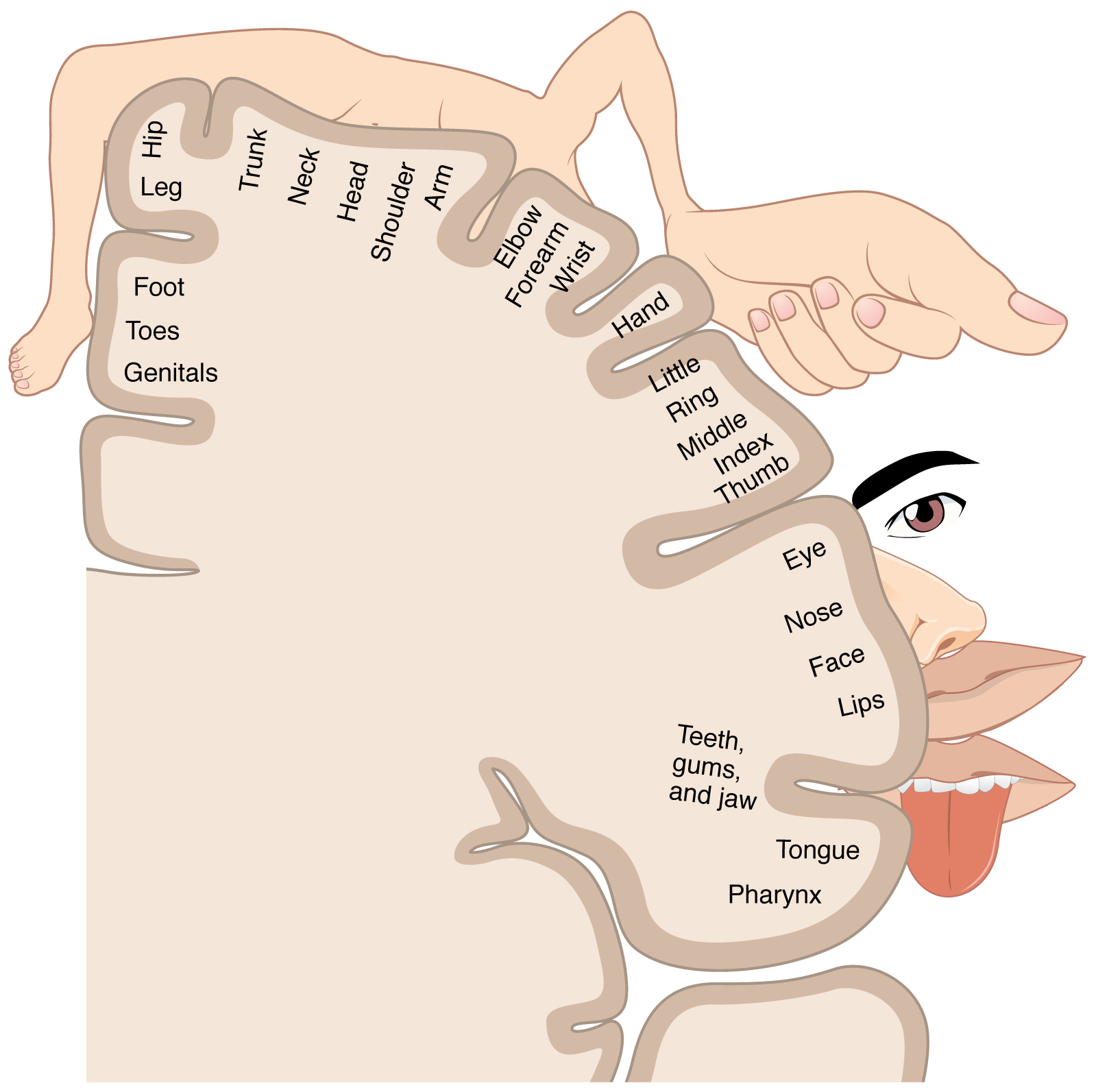
Sensory Homunculus. The illustration is a sensory homunculus of the primary somatosensory cortex.
OpenStax, Public Domain, via Wikimedia Commons
References
Jacobs R, Bou Serhal C, van Steenberghe D. Oral stereognosis: a review of the literature. Clinical oral investigations. 1998 Mar:2(1):3-10 [PubMed PMID: 9667147]
Bajwa H, Al Khalili Y. Physiology, Vibratory Sense. StatPearls. 2024 Jan:(): [PubMed PMID: 31194428]
Dall'Orso S, Steinweg J, Allievi AG, Edwards AD, Burdet E, Arichi T. Somatotopic Mapping of the Developing Sensorimotor Cortex in the Preterm Human Brain. Cerebral cortex (New York, N.Y. : 1991). 2018 Jul 1:28(7):2507-2515. doi: 10.1093/cercor/bhy050. Epub [PubMed PMID: 29901788]
Navarro-Orozco D, Bollu PC. Neuroanatomy, Medial Lemniscus (Reils Band, Reils Ribbon). StatPearls. 2024 Jan:(): [PubMed PMID: 30252296]
Nguyen JD, Duong H. Neurosurgery, Sensory Homunculus. StatPearls. 2024 Jan:(): [PubMed PMID: 31751031]
Roux FE, Djidjeli I, Durand JB. Functional architecture of the somatosensory homunculus detected by electrostimulation. The Journal of physiology. 2018 Mar 1:596(5):941-956. doi: 10.1113/JP275243. Epub 2018 Jan 19 [PubMed PMID: 29285773]
Kumar A, Wroten M. Agnosia. StatPearls. 2024 Jan:(): [PubMed PMID: 29630208]
Veronelli L,Ginex V,Dinacci D,Cappa SF,Corbo M, Pure associative tactile agnosia for the left hand: clinical and anatomo-functional correlations. Cortex; a journal devoted to the study of the nervous system and behavior. 2014 Sep; [PubMed PMID: 25046697]
Level 3 (low-level) evidenceWalker HK, Hall WD, Hurst JW, Bigley GK. Sensation. Clinical Methods: The History, Physical, and Laboratory Examinations. 1990:(): [PubMed PMID: 21250231]
Azhary H, Farooq MU, Bhanushali M, Majid A, Kassab MY. Peripheral neuropathy: differential diagnosis and management. American family physician. 2010 Apr 1:81(7):887-92 [PubMed PMID: 20353146]
Ohno K, Saito Y, Togawa M, Shinohara Y, Ito T, Sugano H, Itamura S, Nishimura Y, Tamasaki A, Maegaki Y. Evolution of a symptomatic diffuse developmental venous anomaly with progressive cerebral atrophy in an atypical case of Sturge-Weber syndrome. Brain & development. 2015 Sep:37(8):817-21. doi: 10.1016/j.braindev.2014.12.003. Epub 2014 Dec 26 [PubMed PMID: 25547041]
Level 3 (low-level) evidenceMeyer S,De Bruyn N,Krumlinde-Sundholm L,Peeters A,Feys H,Thijs V,Verheyden G, Associations Between Sensorimotor Impairments in the Upper Limb at 1 Week and 6 Months After Stroke. Journal of neurologic physical therapy : JNPT. 2016 Jul; [PubMed PMID: 27214520]
Abela E, Missimer JH, Pastore-Wapp M, Krammer W, Wiest R, Weder BJ. Early prediction of long-term tactile object recognition performance after sensorimotor stroke. Cortex; a journal devoted to the study of the nervous system and behavior. 2019 Jun:115():264-279. doi: 10.1016/j.cortex.2019.01.018. Epub 2019 Feb 7 [PubMed PMID: 30875614]
Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW, American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020 Mar 3:141(9):e139-e596. doi: 10.1161/CIR.0000000000000757. Epub 2020 Jan 29 [PubMed PMID: 31992061]
Turville ML, Cahill LS, Matyas TA, Blennerhassett JM, Carey LM. The effectiveness of somatosensory retraining for improving sensory function in the arm following stroke: a systematic review. Clinical rehabilitation. 2019 May:33(5):834-846. doi: 10.1177/0269215519829795. Epub 2019 Feb 25 [PubMed PMID: 30798643]
Level 1 (high-level) evidenceTurville M,Carey LM,Matyas TA,Blennerhassett J, Change in Functional Arm Use Is Associated With Somatosensory Skills After Sensory Retraining Poststroke. The American journal of occupational therapy : official publication of the American Occupational Therapy Association. 2017 May/Jun; [PubMed PMID: 28422633]
Winward CE, Halligan PW, Wade DT. Somatosensory recovery: a longitudinal study of the first 6 months after unilateral stroke. Disability and rehabilitation. 2007 Feb 28:29(4):293-9 [PubMed PMID: 17364779]
Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, Deruyter F, Eng JJ, Fisher B, Harvey RL, Lang CE, MacKay-Lyons M, Ottenbacher KJ, Pugh S, Reeves MJ, Richards LG, Stiers W, Zorowitz RD, American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2016 Jun:47(6):e98-e169. doi: 10.1161/STR.0000000000000098. Epub 2016 May 4 [PubMed PMID: 27145936]
De Bruyn N, Essers B, Thijs L, Van Gils A, Tedesco Triccas L, Meyer S, Alaerts K, Verheyden G. Does sensorimotor upper limb therapy post stroke alter behavior and brain connectivity differently compared to motor therapy? Protocol of a phase II randomized controlled trial. Trials. 2018 Apr 20:19(1):242. doi: 10.1186/s13063-018-2609-4. Epub 2018 Apr 20 [PubMed PMID: 29678195]
Level 1 (high-level) evidence