Introduction
Testicular neoplasm is one of the most common causes of testicular mass. It occurs in approximately 5 per 100,000 men, mainly in the age group of 15-34 years. Seminoma is a malignant germ cell tumor that involves most commonly the testicle or less frequently the mediastinum, the retroperitoneum, or other extra-gonadal sites. It is one of the treatable and curable cancers, with a survival rate of over 95% if discovered in early stages.[1][2][3][4]
Testicular germ cell tumors (GCTs) have different pathological subtypes, including seminoma, teratoma, choriocarcinoma, embryonal, and yolk sac carcinoma. The most significant clinical distinction is between seminoma and nonseminoma, two broad categories with different treatment algorithms. Seminoma based on classification is pure seminoma upon histopathological review. The presence of any nonseminatous elements (even if seminoma is prevalent) changes the classification to nonseminoma.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The exact etiology of seminoma remains undetermined. However, the following factors are associated with an increase in the risk of seminoma:
- History of Cryptorchidism: Risk of seminoma increases with the history of cryptorchidism. There is 10 to 40 times higher risk in patients with an undescended testis, 10% of the patients with cryptorchidism develop germ cell tumor.[5] An abdominal testis usually develops seminoma while a testis surgically brought to the scrotum by orchiopexy is a non-seminomatous germ cell tumor.
- Environmental Exposure: Exposure to chemical compounds like organochlorines, polychlorinated biphenyls, polyvinyl chlorides, phthalates, marijuana, and tobacco is associated with an increased risk of seminoma or other germ cell tumors.[6][7]
- Infections: History of mumps viral infection is related to increased risk of germ cell tumors.[8]
- Others: Other factors include trauma, maternal estrogen exposure, family history of testicular tumors, intersex syndromes (insensitive androgen syndrome and gonadal dysgenesis), and history of cancer in the contralateral testicle.[9]
- Genetics: Genetic changes in the form of amplification and deletions are seen in 12p11.2-p12.1 chromosomal regions. Coffey and colleagues demonstrated that only seminomas contain activating mutations of the KIT gene.[10]
Epidemiology
Testicular germ cell tumors (GCTs) account for the most common malignancy in men aged 15 to 34 years. However, it accounts for less than 1% of all male tumors. The incidence of testicular tumors is rising from the past 20 years. In the United States, testicular seminoma is the most common subtype of testicular cancer.[11] A higher incidence of seminoma is seen among Whites than in African Americans, and the rate has increased in the White population over recent decades.[12]
Pathophysiology
Testicular seminoma is a germ cell neoplasm originating from the seminiferous tubules, due to malignant transformation of primordial germ cells. The exact molecular derangements underlying this transformation are not clearly understood, but the most common genetic finding is the gain of genetic material from chromosome 12p. Some of the mutations noticed in germ cell tumors include BRAF, KIT, KRAS, NRAS, and TP53, although single-gene mutations are relatively uncommon.[13]
Seminomas can be subdivided into one of three categories based on histology: classic, anaplastic, and spermatocytic. In testicular seminoma, alpha-fetoprotein (AFP) is in the normal range. If AFP is elevated and the presence of noseminomatous elements in histopathological specimens makes the diagnosis of nonseminotaous germ cell tumor. Germ cell carcinoma in situ (CIS) is a premalignant condition with the propensity of progression to seminoma or embryonal cancer. Patients with cryptorchidism, infertility, prior contralateral germ cell tumors, intersex disorders, or atrophic testes more commonly have CIS. Testicular microcalcifications observed on scrotal ultrasound were implicated in the testicular carcinoma development.
Histopathology
Histological Findings
Most commonly, there is a diffuse arrangement of pale cells that is interrupted by fibrovascular septa containing lymphocytes. The tumor cells characteristically have pale to clear cytoplasm, with crisp cytoplasmic membranes and polygonal nuclei with finely granular chromatin and frequently flattened edges. One or more large, centrally located nucleoli are present. The abundant cytoplasm results in relatively evenly spaced, non-overlapping nuclei in most cases. The cytoplasmic clarity is attributable to abundant glycogen particles demonstrable with the periodic acid-Schiff stain. Less commonly, the cytoplasm is denser, and the nuclei more crowded. This may result in a plasmacytoid appearance. A variably prominent lymphocytic infiltrate occurs in almost every case and a granulomatous reaction in more than half. Approximately 10-20% of tumors contain admixed syncytiotrophoblast cells that vary in prominence from widely scattered to prominent aggregates.[14]
Immunohistochemical Findings
Seminomas stain for antigens characteristic of immature, fetal-type germ cells (gonocytes), including placental alkaline phosphatase (86-95%; cytoplasmic membrane), KIT (90-100%; cytoplasmic membrane), OCT3/4 (100%; nuclear), SALL4 (100%; nuclear), and SOX17 (95%; nuclear). Podoplanin is also positive (100%; cytoplasmic membrane). Cytokeratin AE1/AE3 immunoreactivity varies (20-36%), but is often negative or stains the cytoplasm of only a minority of tumor cells, often in a para nuclear dot-like pattern. CD30 is characteristically negative, as is epithelial membrane antigen (2%). Stains for alpha-fetoprotein are always negative.
History and Physical
Seminoma usually presents as a nodule or painless swelling of the testis. It may be an incidental finding by the patients or his partner. Patients sometimes complain of a dull ache or heavy sensation in the lower abdomen, perineal area, or scrotum. Rarely, acute pain or hematospermia may be the presenting symptoms. Cases of advanced disease, with metastases, present as a neck mass (cervical or supraclavicular lymph node metastases), cough or dyspnea (pulmonary metastases), anorexia, nausea, vomiting, or gastrointestinal hemorrhage (gastroduodenal metastases), bone pain (skeletal metastases), central or peripheral nerve involvement (cerebral, spinal cord, or peripheral nerve involvement). History of cryptorchidism and orchiopexy is noted in some cases.
Evaluation
In patients with a suspected testicular mass, initial workup includes laboratory tests and imaging results. Serum tumor marker levels, including alpha-fetoprotein (AFP), beta-human chorionic gonadotropin (beta-HCG), and lactate dehydrogenase (LDH), are measured. Serum beta-HCG and LDH levels can be elevated in seminomas while AFP is not raised in pure seminomas. LDH is a less specific marker, but levels can correlate with overall tumor burden. Beta-HCG levels are increased in 5-10% of patients with seminoma, and levels may correlate with metastatic disease but not with overall survival. Placenta-like alkaline phosphatase may be elevated in patients with seminoma, especially when the tumor burden increases; however, these may also be increased with smoking.
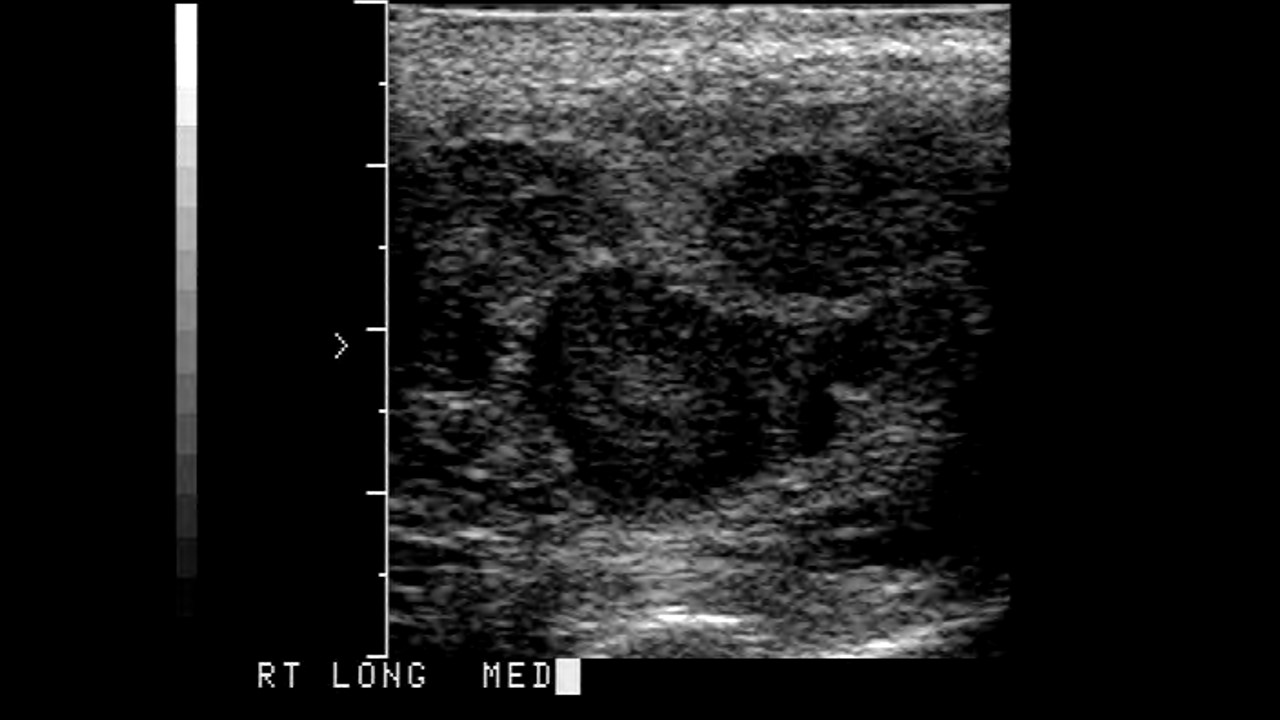
Scrotal ultrasonography is done to rule out other conditions. It shows a homogeneous hypoechoic intratesticular mass, more extensive lesions may be inhomogeneous. Cystic areas and calcifications are less common in seminomas than in non-seminomatous tumors. For a definitive diagnosis, an orchiectomy is performed, which is diagnostic as well as therapeutic. Histologic and laboratory results confirm the diagnosis and help differentiate the type of testicular cancers.
Once the diagnosis is confirmed, a chest x-ray or chest CT scan, abdominal and pelvic CT scan, MRI brain, and bone scan may be done to look for metastases depending upon the stage and signs and symptoms of metastases. PET can be used to assess disease activity following chemotherapy treatment and to identify recurrences.[15]
The most commonly used staging systems for germ cell tumors are those proposed by the Americal Joint Commission on Cancer (AJCC) and the International Union Against Cancer (IUAC).
Risk stratification of seminomas is not dependent on serum tumor markers (unlike non-seminomatous germ cell tumors) but rather based on the presence or absence of nonpulmonary visceral metastases.[16]
Treatment / Management
Radical orchiectomy provides both diagnostic and therapeutic implications in seminoma. It is indicated for all stages of the disease. Adjuvant chemotherapy or radiotherapy depends upon the clinical staging that follows radical inguinal orchiectomy. Generally, treatment plans are as follows:[17][18][19]
- Stage I: For patients with stage I seminoma, orchiectomy is usually curative.[20][21] Options for post-orchiectomy management include surveillance alone or adjuvant chemotherapy with one single infusion of carboplatin or prophylactic radiotherapy (declining interest).
- Stage II: Following radical inguinal orchiectomy, the treatment of stage II diseases depends on the extent of the lymph node involvement. Radiotherapy with or without cisplatin-based chemotherapy is recommended.
- Stage III: For stage III diseases, chemotherapy with bleomycin, etoposide, and cisplatin (BEP) or etoposide and cisplatin (EP) is preferred. Radiotherapy may be indicated in select cases.[22] In patients with an intermediate prognosis, it might be hard to tolerate bleomycin, and therefore the addition of ifosfamide to etoposide and cisplatin (VIP) is reasonable.[23] (B3)
After treatment patient requires life long follow up. Surveillance includes the following, with the frequency determined by the disease stage and duration of follow up.
- History and physical examination
- Serum tumor markers ( beta-hCG, LDH, AFP)
- Chest radiography
- CT scan of the abdomen, with or without CT scan of the pelvis
Differential Diagnosis
Histological differential diagnosis of seminoma include:
- Non-seminomatous germ cell tumor, including embryonal carcinoma, cholangiocarcinoma, yolk sac tumor or teratoma
- Leydig and Sertoli cell tumors
- Granulosa cell tumors
- Gonadoblastoma
- Lymphoma
- Mesothelioma
- Adenocarcinoma of the rete testis
- Epidermoid cyst
- Metastatic carcinoma
- Epididymitis
- Hydrocele
Surgical Oncology
Radical inguinal orchiectomy is the standard procedure for diagnostic and therapeutic purposes. Trans scrotal orchiectomy or biopsy of the testicular mass is contraindicated for the risk of tumor seeding of the lymphatic drainage. Spinal or general anesthesia may be used. An inguinal incision is made to expose the external and internal iliac canal. External iliac fascia is opened, revealing the spermatic cord. The spermatic cord is controlled to stop retroperitoneal lymphatic and venous drainage of tumor cells. After that, deliver the testis from the scrotum and ligate vas deferens and spermatic arteries separately. Retroperitoneal lymph node dissection is done if necessary. Reapproximate the external oblique fascia and close the skin in standard fashion. Conduct a follow-up study by staging and referring the patient for appropriate adjuvant therapies.
Staging
In the TNM classification system, assessments of the primary tumor (T), lymph node involvement (N), and distant metastases (M) are combined with serum tumor marker values (S) like AFP, beta-HCG, and LDH to define prognostic stage groups from I to III. The treatment plan, including chemotherapy and radiotherapy, depends upon the stage of the tumor diagnosed.
AJCC TNM staging classification 8th ed., 2017[24]
Definitions for T, N, M:
Pathological T
- Tx Primary tumor cannot be assessed
- T0 No evidence of primary tumor
- Tis Germ cell neoplasia in situ
- T1 Tumor limited to the testicle (including rete testis invasion) without lymphovascular invasion
- T1a Tumor smaller than 3 cm in size
- T1b Tumor 3 cm or larger
- T2 Tumor limited to the testicle (including rete testis invasion) with lymphovascular invasion or tumor invading hilar soft tissue or epididymis or penetrating visceral mesothelial layer covering the external surface of tunica albuginea with or without lymphovascular invasion
- T3 Tumor directly invades spermatic cord soft tissue with or without lymphovascular invasion
- T4 Tumor invades scrotum with or without lymphovascular invasion
Clinical N
- Nx Regional lymph nodes cannot be assessed
- N0 No regional lymph node metastasis
- N1 Metastasis with a lymph node mass 2 cm or smaller in greatest dimension or multiple lymph nodes, none larger than 2 cm in greatest dimension
- N2 Metastasis with a lymph node mass larger than 2 cm but not larger than 5 cm in greatest dimension or multiple lymph nodes, if any mass larger than 2 cm but not larger than 5 cm in greatest dimension
- N3 Metastasis with a lymph node mass larger than 5 cm in greatest dimension
Pathological N
- Nx Regional lymph nodes cannot be assessed
- N0 No regional lymph node metastasis
- N1 Metastasis with a lymph node mass 2 cm or smaller in greatest dimension and less than or equal to five nodes positive, none larger than 2 cm in greatest dimension
- N2 Metastasis with a lymph node mass larger than 2 cm but not larger than 5 cm in greatest dimension; or more than five nodes positive, none larger than 5 cm; or evidence of extranodal extension of tumor
- N3 Metastasis with a lymph node mass larger than 5 cm in greatest dimension
Clinical or pathological M
- M0 No distant metastases
- M1 Distant metastases
- M1a Non-retroperitoneal nodal or pulmonary metastases
- M1b Non-pulmonary visceral metastases
Serum markers S
- Sx Marker studies not available or not performed
- S0 Marker study levels within normal limits
- S1 LDH <1.5 x N* and hCG (mIU/mL) <5,000 and AFP (ng/mL) <1,000
- S2 LDH 1.5–10 x N* or hCG (mIU/mL) 5,000–50,000 or AFP (ng/mL) 1,000 to 10,000
- S3 LDH >10 x N* or hCG (mIU/mL) >50,000 or AFP (ng/mL) >10,000
AJCC prognostic stage groups
- Stage 0 pTis N0 M0 S0
- Stage I pT1-T4 N0 M0 Sx
- Stage IA pT1 N0 M0 S0
- Stage IB pT2 N0 M0 S0 pT3 N0 M0 S0 pT4 N0 M0 S0
- Stage IS Any pT/Tx N0 M0 S1-3
- Stage II Any pT/Tx N1-3 M0 Sx
- Stage IIA Any pT/Tx N1 M0 S0 Any pT/Tx N1 M0 S1
- Stage IIB Any pT/Tx N2 M0 S0 Any pT/Tx N2 M0 S1
- Stage IIC Any pT/Tx N3 M0 S0 Any pT/Tx N3 M0 S1
- Stage III Any pT/Tx Any N M1 SX
- Stage IIIA Any pT/Tx Any N M1a S0 Any pT/Tx Any N M1a S1
- Stage IIIB Any pT/Tx N1-3 M0 S2 Any pT/Tx Any N M1a S2
- Stage IIIC Any pT/Tx N1-3 M0 S3 Any pT/Tx Any N M1a S3 Any pT/Tx Any N M1b Any S
*Serum tumor marker values (S) are measured after orchiectomy.
Prognosis
According to information from the Surveillance, Epidemiology, and Rend-Results (SEER) database, as published on the American Cancer Society webpage, the 5-year relative survival rates for testicular cancer are;
- Localized disease (stage I) 99%
- Regional disease (stage II) 96%
- Distant disease (Stage III) 73%
Patients with testicular cancer are at an increased risk of secondary cancers because of their young age at diagnosis, high cure rate, and exposure to radiation, chemotherapy, or both. Tumors included malignant mesothelioma and those of the lung, colon, bladder, pancreas, and stomach.
Complications
Both radiation therapy and chemotherapy have long-term complications in survivors of testicular cancer. With post-therapy life estimates being approximately more than 40 years in these young individuals, the morbidity of long term complications can be very significant.
- Chemotherapy related neurotoxicity: Peripheral neuropathy is a common adverse event of platinum chemotherapy. About 20 to 40% of patients will develop neurotoxicity, which can be painful and also cause permanent sensory abnormalities.
- Cardiovascular complications: There can be up to a seven-fold increased risk of developing a cardiac event in survivors of testicular cancer who received cisplatin-based chemotherapy.[25]
- Infertility and hypogonadism: Chemotherapy and the use of radiation to seminoma affected scrotum can result in oligospermia and infertility. Thus, sperm banking must be offered to all patients before undergoing chemotherapy or radiotherapy. The risk of infertility is directly proportional to the intensity and duration of the treatment. Hypogonadism or testicular dysfunction is a common sequela among survivors of testicular cancer.
- Ototoxicity: Cisplatin can cause permanent, sensorineural hearing loss and tinnitus.
- Secondary neoplasms: Both chemotherapy and radiotherapy can increase the risk of secondary malignant tumors in survivors. Etoposide can cause treatment-related leukemia, and risk is proportional to the total dose administered.
Postoperative and Rehabilitation Care
Following radical inguinal orchiectomy, postoperative reconstructive surgery with prosthetic testicle is gaining popularity. The operation is comparable to an inguinal herniorrhaphy, and the patient should do limited physical activity for a brief post-surgery period. A short course of pain management medication is advised. Complications are rare but can include wound infection, hematoma, anesthetic risk, and inguinal skin numbness.
Surveillance for seminomas after initial management is as follows:
Clinical stage I seminoma after orchiectomy alone:
- Year 1: History and physical examination (H&P) every 3-6 months, CT scan of abdomen +/- pelvis at 3, 6, and 12 months.
- Years 2 and 3: H&P and CT scan of abdomen +/- pelvis every 6-12 months.
- Years 4 and 5: H&P annually, and CT scan of abdomen +/- pelvis every 12-24 months.
*At any point in five years, a chest x-ray is recommended as clinically indicated, and chest CT with contrast is obtained if the patient is symptomatic. Serum tumor markers are optional with H&P.
Clinical stage I seminoma after adjuvant therapy (chemotherapy or radiation):
- Years 1 and 2: H&P every 6-12 months, and CT scan of abdomen +/- pelvis annually
- Year 3: H&P and CT of abdomen +/- pelvis annually
- Years 4 and 5: H&P annually
*At any point in five years, a chest x-ray is recommended as clinically indicated, and chest CT with contrast is obtained if the patient is symptomatic. Serum tumor markers are optional with H&P.
Clinical stage IIA and non-bulky IIB seminoma after radiation or chemotherapy:
- Year 1: H&P every three months, CT scan of abdomen +/- pelvis at three months and then every 6-12 months, and chest x-ray every six months.
- Year 2: H&P every six months, CT scan of abdomen +/- pelvis annually, and chest x-ray every six months.
- Year 3: H&P every six months, and CT scan of abdomen +/- pelvis annually.
- Year 4 and 5: H&P every six months and CT scan fo abdomen +/- pelvis as clinically indicated.
*Serum tumor markers are optional with H&P.
Clinical bulky stage IIB, IIC, and stage III seminoma after chemotherapy:
- Year 1: H&P with serum tumor markers every two months, CT scan of abdomen/pelvis every four months, and chest X-ray every two months.
- Year 2: H&P with serum tumor markers every three months, CT scan of abdomen/pelvis every six months, and chest X-ray every three months.
- Years 3 and 4: H&P with serum tumor markers every six months, CT scan of abdomen/pelvis chest X-ray annually.
- Year 5: H&P with serum tumor markers annually, CT scan of abdomen/pelvis as clinically indicated, and chest X-ray annually.
Consultations
Diagnosis and management of seminoma need a multidisciplinary approach and teamwork. Following specialties should work in coordination for better management
- Urology
- Surgical oncology
- Radiation oncology
- Medical oncology
- Radiology
- Histopathology
- Cosmetic surgery
- Fertility medicine
Deterrence and Patient Education
There is no useful screening test for the detection of testicular cancer. Hence, the young population needs to be aware of the importance of self-examination. Early detection of the disease has an excellent prognosis. Therefore, self-examination should be encouraged in the patient with a positive family history of testicular cancer or a history of cryptorchidism.
Sperm banking should be discussed in all patients of reproductive age. The discussion should take place before the patient undergoes any procedure that may compromise fertility, including orchiectomy, chemotherapy, or radiotherapy. If the patient desires sperm banking, it may be performed before or after surgery, but must be completed before radiation therapy or chemotherapy.
Enhancing Healthcare Team Outcomes
Timely diagnosis and management of testicular cancers result in excellent prognosis and near-normal life expectancy. Therefore, it is of utmost importance for ER providers and primary care providers to have a low threshold for evaluating testicular symptoms in young patients, with a complete history and physical examination and urgent testicular ultrasound. Based on the results of the ultrasound and level of clinical suspicion, an urgent referral or consultation with a urologist is needed. Once orchiectomy is performed, the patient must be followed by a multi-disciplinary oncology team involving medical oncologists, radiation oncologists, and surgical oncologists to discuss staging, adjuvant therapies, and surveillance.
Media
References
Bokemeyer C, Nichols CR, Droz JP, Schmoll HJ, Horwich A, Gerl A, Fossa SD, Beyer J, Pont J, Kanz L, Einhorn L, Hartmann JT. Extragonadal germ cell tumors of the mediastinum and retroperitoneum: results from an international analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002 Apr 1:20(7):1864-73 [PubMed PMID: 11919246]
Level 2 (mid-level) evidenceDieckmann KP, Richter-Simonsen H, Kulejewski M, Ikogho R, Zecha H, Anheuser P, Pichlmeier U, Isbarn H. Testicular Germ-Cell Tumours: A Descriptive Analysis of Clinical Characteristics at First Presentation. Urologia internationalis. 2018:100(4):409-419. doi: 10.1159/000488284. Epub 2018 Apr 12 [PubMed PMID: 29649815]
Chung P, Warde P. Testicular cancer: seminoma. BMJ clinical evidence. 2011 Jan 25:2011():. pii: 1807. Epub 2011 Jan 25 [PubMed PMID: 21477387]
Level 1 (high-level) evidenceFung C, Dinh PC, Fossa SD, Travis LB. Testicular Cancer Survivorship. Journal of the National Comprehensive Cancer Network : JNCCN. 2019 Dec:17(12):1557-1568. doi: 10.6004/jnccn.2019.7369. Epub [PubMed PMID: 31805527]
Batool A, Karimi N, Wu XN, Chen SR, Liu YX. Testicular germ cell tumor: a comprehensive review. Cellular and molecular life sciences : CMLS. 2019 May:76(9):1713-1727. doi: 10.1007/s00018-019-03022-7. Epub 2019 Jan 22 [PubMed PMID: 30671589]
Barchi M, Innocenzi E, Giannattasio T, Dolci S, Rossi P, Grimaldi P. Cannabinoid Receptors Signaling in the Development, Epigenetics, and Tumours of Male Germ Cells. International journal of molecular sciences. 2019 Dec 18:21(1):. doi: 10.3390/ijms21010025. Epub 2019 Dec 18 [PubMed PMID: 31861494]
Gurney J, Shaw C, Stanley J, Signal V, Sarfati D. Cannabis exposure and risk of testicular cancer: a systematic review and meta-analysis. BMC cancer. 2015 Nov 11:15():897. doi: 10.1186/s12885-015-1905-6. Epub 2015 Nov 11 [PubMed PMID: 26560314]
Level 1 (high-level) evidenceGarolla A, Vitagliano A, Muscianisi F, Valente U, Ghezzi M, Andrisani A, Ambrosini G, Foresta C. Role of Viral Infections in Testicular Cancer Etiology: Evidence From a Systematic Review and Meta-Analysis. Frontiers in endocrinology. 2019:10():355. doi: 10.3389/fendo.2019.00355. Epub 2019 Jun 12 [PubMed PMID: 31263452]
Level 1 (high-level) evidenceFukawa T, Kanayama HO. Current knowledge of risk factors for testicular germ cell tumors. International journal of urology : official journal of the Japanese Urological Association. 2018 Apr:25(4):337-344. doi: 10.1111/iju.13519. Epub 2018 Jan 17 [PubMed PMID: 29345008]
Coffey J, Linger R, Pugh J, Dudakia D, Sokal M, Easton DF, Timothy Bishop D, Stratton M, Huddart R, Rapley EA. Somatic KIT mutations occur predominantly in seminoma germ cell tumors and are not predictive of bilateral disease: report of 220 tumors and review of literature. Genes, chromosomes & cancer. 2008 Jan:47(1):34-42 [PubMed PMID: 17943970]
Ghazarian AA, Trabert B, Devesa SS, McGlynn KA. Recent trends in the incidence of testicular germ cell tumors in the United States. Andrology. 2015 Jan:3(1):13-8. doi: 10.1111/andr.288. Epub 2014 Oct 20 [PubMed PMID: 25331158]
Nigam M, Aschebrook-Kilfoy B, Shikanov S, Eggener S. Increasing incidence of testicular cancer in the United States and Europe between 1992 and 2009. World journal of urology. 2015 May:33(5):623-31. doi: 10.1007/s00345-014-1361-y. Epub 2014 Jul 17 [PubMed PMID: 25030752]
Level 2 (mid-level) evidenceSheikine Y, Genega E, Melamed J, Lee P, Reuter VE, Ye H. Molecular genetics of testicular germ cell tumors. American journal of cancer research. 2012:2(2):153-67 [PubMed PMID: 22432056]
Tourne M, Radulescu C, Allory Y. [Testicular germ cell tumors: Histopathological and molecular features]. Bulletin du cancer. 2019 Apr:106(4):328-341. doi: 10.1016/j.bulcan.2019.02.004. Epub 2019 Mar 21 [PubMed PMID: 30905378]
Zores T, Mouracade P, Duclos B, Saussine C, Lang H, Jacqmin D. [Surveillance of stage I testicular seminoma: 20 years oncological results]. Progres en urologie : journal de l'Association francaise d'urologie et de la Societe francaise d'urologie. 2015 Apr:25(5):282-7. doi: 10.1016/j.purol.2015.01.009. Epub 2015 Feb 25 [PubMed PMID: 25724863]
Level 2 (mid-level) evidenceInternational Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1997 Feb [PubMed PMID: 9053482]
Level 2 (mid-level) evidenceStokes W, Amini A, Maroni PD, Kessler ER, Stokes C, Cost CR, Greffe BS, Garrington TP, Liu AK, Cost NG. Patterns of care and survival outcomes for adolescent and young adult patients with testicular seminoma in the United States: A National Cancer Database analysis. Journal of pediatric urology. 2017 Aug:13(4):386.e1-386.e7. doi: 10.1016/j.jpurol.2016.12.009. Epub 2017 Jan 17 [PubMed PMID: 28153774]
Chovanec M, Hanna N, Cary KC, Einhorn L, Albany C. Management of stage I testicular germ cell tumours. Nature reviews. Urology. 2016 Nov:13(11):663-673. doi: 10.1038/nrurol.2016.164. Epub 2016 Sep 13 [PubMed PMID: 27618772]
Motzer RJ, Jonasch E, Agarwal N, Beard C, Bhayani S, Bolger GB, Chang SS, Choueiri TK, Costello BA, Derweesh IH, Gupta S, Hancock SL, Kim JJ, Kuzel TM, Lam ET, Lau C, Levine EG, Lin DW, Michaelson MD, Olencki T, Pili R, Plimack ER, Rampersaud EN, Redman BG, Ryan CJ, Sheinfeld J, Shuch B, Sircar K, Somer B, Wilder RB, Dwyer M, Kumar R. Testicular Cancer, Version 2.2015. Journal of the National Comprehensive Cancer Network : JNCCN. 2015 Jun:13(6):772-99 [PubMed PMID: 26085393]
Faouzi S, Ouguellit S, Loriot Y. [Stage 1 germ-cell tumour]. Bulletin du cancer. 2019 Oct:106(10):887-895. doi: 10.1016/j.bulcan.2019.03.010. Epub 2019 May 12 [PubMed PMID: 31088678]
Stephenson A, Eggener SE, Bass EB, Chelnick DM, Daneshmand S, Feldman D, Gilligan T, Karam JA, Leibovich B, Liauw SL, Masterson TA, Meeks JJ, Pierorazio PM, Sharma R, Sheinfeld J. Diagnosis and Treatment of Early Stage Testicular Cancer: AUA Guideline. The Journal of urology. 2019 Aug:202(2):272-281. doi: 10.1097/JU.0000000000000318. Epub 2019 Jul 8 [PubMed PMID: 31059667]
Alsdorf W,Seidel C,Bokemeyer C,Oing C, Current pharmacotherapy for testicular germ cell cancer. Expert opinion on pharmacotherapy. 2019 May; [PubMed PMID: 30849243]
Level 3 (low-level) evidenceFizazi K, Delva R, Caty A, Chevreau C, Kerbrat P, Rolland F, Priou F, Geoffrois L, Rixe O, Beuzeboc P, Malhaire JP, Culine S, Aubelle MS, Laplanche A. A risk-adapted study of cisplatin and etoposide, with or without ifosfamide, in patients with metastatic seminoma: results of the GETUG S99 multicenter prospective study. European urology. 2014 Feb:65(2):381-6. doi: 10.1016/j.eururo.2013.09.004. Epub 2013 Sep 13 [PubMed PMID: 24094847]
Level 3 (low-level) evidenceAmin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA: a cancer journal for clinicians. 2017 Mar:67(2):93-99. doi: 10.3322/caac.21388. Epub 2017 Jan 17 [PubMed PMID: 28094848]
Huddart RA, Norman A, Shahidi M, Horwich A, Coward D, Nicholls J, Dearnaley DP. Cardiovascular disease as a long-term complication of treatment for testicular cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003 Apr 15:21(8):1513-23 [PubMed PMID: 12697875]
Level 2 (mid-level) evidence