Introduction
Pulmonary function tests (PFTs) allow physicians to evaluate the respiratory function of their patients in many clinical situations and when there are risk factors for lung disease, occupational exposures, and pulmonary toxicity. National guidelines for the measurements and interpretation of PFT are regularly updated, and the most recent guidelines developed by the international joint Task force from the European Respiratory Society and the American Thoracic Society (EUR/ATS) were published in 2022.[1]
The results of the PFTs are affected by the effort of the patient. PFTs do not provide a specific diagnosis; the results should be combined with relevant history, physical exam, and laboratory data to help reach a diagnosis. PFTs also allow physicians to quantify the severity of the pulmonary disease, follow it up over time, and assess its response to treatment.[2]
Procedures
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Procedures
Spirometry
Spirometry is a physiological test that measures the ability to inhale and exhale air relative to time. Spirometry is a diagnostic test of several common respiratory disperses such as asthma and chronic obstructive pulmonary disease (COPD). It is also instrumental in monitoring the progression of various respiratory disorders. The main results of spirometry are forced vital capacity (FVC), forced expiratory volume exhaled in the first second (FEV1), and the FEV1/FVC ratio. The procedure of spirometry has 3 phases: 1) maximal inspiration; 2) a “blast” of exhalation; 3) continued complete exhalation to the end of the test. There are within-maneuver acceptability and between-maneuver reproducibility criteria for spirometry (Table 2).
The spirometry procedure is usually performed in a standard sitting position. However, spirometry measurement in the supine position may be indicated in certain neuromuscular disorders. For example, in individuals with spinal cord injury, at the level of T6 and above, the FVC and FEV1 are decreased and become lower in the supine position compared to the upright position, suggesting an increased risk of hypoventilation during sleep.[3]
Lung Volume
The measurement of lung volumes includes several important variables, such as functional reserve capacity (FRC), vital capacity (VC), slow vital capacity (SVC), expiratory reserve volume (ERV), and residual volume (RV). The lung volume measurement is very important to detect changes in lung volume independent of effort, especially when FVC is reduced on spirometry.
FRC is the volume of the amount of gas in the lungs at the end of expiration during tidal breathing. FRC is also the sum of ERV and RV. Once FRC has been measured, all other volumes can be calculated.
ERV is the volume of gas maximally exhaled after end-inspiratory tidal breathing. RV is the volume of gas in the airways after a maximal exhalation. VC is the volume of gas expelled from full inspiration to residual volume. The FVC is similar, but the patient exhales at maximal speed and effort.
In addition to RV and ERV, there is a tidal volume (TV) and inspiratory reserve volume (IRV). IRV is the volume of gas that can be maximally inhaled from the end-inspiratory tidal breathing. TV is the volume of gas inhaled or exhaled with each breath at rest. Slow vital capacity (SVC) can be measured as the maximal amount of air exhaled in a relaxed expiration from full inspiration to residual volume; exhalation should be terminated after 15 seconds.[6] The SVC may be a useful measurement when the FVC is reduced and airway obstruction is present.
Total lung capacity (TLC) is the volume of air in the lungs at the end of maximal inspiration. The sum of RV and VC or FRC and inspiratory capacity (IC) equals TLC, which is the gold standard for diagnosing restrictive lung disease. TLC less than 80% predicted is diagnostic of a restrictive ventilatory defect.[4][5]
There are two methods to measure lung volumes: body plethysmography and gas dilution methods (nitrogen washout and inert gas dilution). The gas dilution method uses an inert gas (poorly soluble in alveolar blood and lung tissues), either nitrogen or helium. The subject breathes a gas mixture until equilibrium is achieved. The volume and mixture of gas exhaled after the equilibrium has been achieved permit the calculation of FRC. In body plethysmography, the subject sits inside a body box and breathes against a shutter valve. FRC is calculated using Boyle's law (at a given temperature, the product of gas volume and pressure is constant). FRC calculated by body plethysmography is usually larger in subjects with obstructive lung disease and air trapping than FRC calculated using gas dilution methods. Body plethysmography is considered the gold standard for lung volume measurement, especially in heterogeneous airflow obstruction, such as in COPD or asthma, where plethysmography is more accurate than helium dilution.[6]
Diffusion Capacity
Diffusion studies the diffusion of gases across the alveolar-capillary membranes. Its measurement uses carbon monoxide (CO) to calculate the pulmonary diffusion capacity. The most common method is the standard single-breath D. It is measured in milliliters per minute per mm Hg.
The factors affecting the D are volume and distribution of ventilation, mixing and diffusion, the composition of the gas, characteristics of the alveolar membrane and lung parenchyma, the volume of alveolar capillary plasma, concentration and binding properties of hemoglobin, and gas tensions in blood entering the alveolar capillaries. A detailed list of factors affecting DLCO is listed in Table 3.
The diffusion capacity depends on multiple factors, and its value should be adjusted. For correct interpretation, specific adjustments should be made for hemoglobin, carboxyhemoglobin, and FiO2. Adjustment for lung volumes is controversial, and further studies are needed.
The DLCO is interpreted in conjunction with spirometry and lung volumes. Table 3 shows the severity classification for DLCO. For example, high DLCO is associated with asthma, obesity, and intrapulmonary hemorrhage. Normal spirometry and lung volumes with low DLCO can be present in pulmonary vascular diseases, early ILD, or emphysema. An obstructive ventilatory defect with low DLCO suggests emphysema or lymphangiomyomatosis.[7][8]
Respiratory Muscle Pressures
The respiratory muscle strength is assessed with maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP). The MIP reveals the strength of the diaphragm and other inspiratory muscles, whereas the MEP indicates the strength of the abdominal and other expiratory muscles. MIP and MEP are measured three times, and maximal value is reported. For adults 18 to 65 years old, MIP should be lower than -90 cmHO in men and -70 cmHO in women. In adults older than 65, MIP should be less than -65 cmH2O in men and -45 cmH2O in women. MEP should be higher than 140 cmH2O in men and 90 cmH2O in women. MEP less than 60 cmH2O predicts a weak cough and difficulty clearing secretions.
Bronchoprovocation testing
There are several types of bronchoprovocation testing available to assess airway responsiveness. The pharmacologic challenge or exercise challenge are the most common types for bronchoprovocation testing and the accurate diagnosis of asthma. Before initiating the bronchoprovocation testing, several precautions should be taken: (1) The person performing the test should be able to identify the occurrence of severe bronchospasm; (2) a rapid-acting beta-agonist (such as albuterol) should be immediately available; (3) FEV should be insured that has returned to or exceeded to baseline before the patient leaves the pulmonary function laboratory; (4) equipment for resuscitation should also be available and accessible with qualified personnel.
The most commonly used agent for bronchoprovocation is methacholine (acetylcholine derivative) at incremental doses ranging from approximately 0.03 mg/mL to 16 mg/mL. Bronchoprovocation testing is usually performed after obtaining baseline spirometry, using a diluent that is aerosolized via nebulizer for at least one minute, then spirometry is repeated twice. The dose of methacholine is then sequentially increased until a decrease in FEV > 20%. The dose of methacholine is associated with a greater than 20% decrease in FEV1 and is referred to as the provocative dose or PD20. A methacholine PD20 of 8 mg/mL or less is considered a positive bronchoprovocation test. However, a PD20 greater than 16 mg/mL is regarded as a negative test. [9]
Six-minute walk test (6MWT)
Six-minute walk test s a standard test to assess exercise capacity objectively and determine prognosis in many respiratory (such as COPD, idiopathic pulmonary fibrosis, and pulmonary hypertension) and non-respiratory conditions (such as heart failure).[10][11] The minimal clinically important difference for change in the 6MWT distance of adults is approximately 30 meters.[12] The 6MWT is not designed to be used for home oxygen titration and assessment, and a separate study is recommended to assess the need and dose of supplemental oxygen. Performance of the test requires the presence of a flat, straight corridor 30 m (100 feet) in length, the ability to monitor heart rate and pulse oximetry throughout the test, and if the patient uses supplemental oxygen, to record the flow rate and type of Oxygen device. [10]
Indications
There are multiple indications to obtain PFTs. Table 1 summarizes the most common indications.
PFTs can be physically demanding for patients, and it is recommended to wait one month after an acute coronary syndrome or myocardial infarction. Other relative contraindications are thoracic/abdominal surgery, brain/eye/ear/otolaryngological surgery, pneumothorax, ascending aortic aneurysm, hemoptysis, pulmonary embolism, severe hypertension (systolic blood pressure greater than 200 mm Hg, diastolic blood pressure greater than 120 mm Hg).[13]
The bronchoprovocation testing is mainly indicated for patients with intermittent asthma symptoms and normal baseline spirometry.
Normal and Critical Findings
Normal findings of spirometry are an FEV1/FVC ratio of greater than 0.70 and both FEV1 and FVC above 80% of the predicted value. If lung volumes are performed, TLC above 80% of the predictive value is normal. Diffusion capacity above 75% of the predicted value is also considered normal.
The natural changes in lung function over time are an important indicator of the health status of the lungs. In non-smokers, FEV1 typically decreases by approximately 30 mL per year. Traditionally a 10% change in FEV1 from baseline over a year duration in healthy individuals is considered significant clinically.[1] However, changes in FEV1 and FVC over time depend on patient demographics such as age, sex, and baseline lung function. Therefore, future studies are needed to adjust for these factors.
Interfering Factors
The results of the PFTs might not be accurate if there is a lack of cooperation or poor understanding of instructions from the patient. Also, if the patient has an acute illness or symptom (for example, altered mental status, nausea, diarrhea, abdominal or chest pain, or cough) are likely to have suboptimal results.
Complications
PFTs are safe in general, and there are no complications. There is some potential harm from 4 key factors:
- Maximal pressures generated in the thorax and their impact on abdominal and thoracic organs/tissues
- Large swings in blood pressure cause stress on tissues in the body
- Expansion of the chest wall and lungs
- Spread of infections (e.g., tuberculosis, hepatitis B, HIV)
Contraindications of PFTs are related to those four factors to prevent potential complications like acute coronary syndrome, rupture of aneurysms, and dehiscence of the surgical wound.[13]
Patients with myocardial infarction, unstable heart disease, or stroke within the previous three months should not perform bronchoprovocation testing. In addition, patients with an FEV <70 % of predicted are usually excluded from performing bronchoprovocation testing.[9]
Clinical Significance
The first step in interpreting PFTs is to review the test quality. Once the quality has been assured, comparisons can be made with healthy subjects and abnormal physiological patterns.
Types of Ventilatory Defects (Table 3)
Obstructive Abnormalities
An obstructive defect is a disproportional decrease in maximal airflow from the lung (FEV1) relative to the maximal volume (FVC) that can be displaced from the lung. In practical terms, an FEV1/FVC ratio of less than 0.70 defines an obstructive ventilatory defect. However, other variables such as age, race, and gender affect this cutoff to estimate expected lung function in health.[1] Hence the decrease in FEV1 and FVC are compared to the lower limit of normal (LLN) to identify whether or not the observed result can be expected in otherwise healthy individuals of similar age, sex, and height.
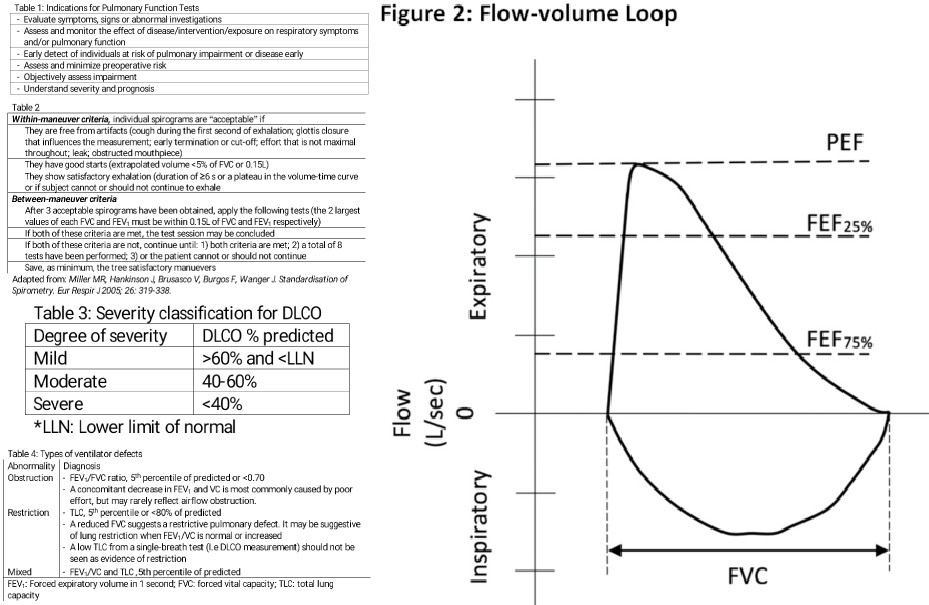
The earliest change associated with an obstruction in small airways is a slowing in the terminal portion of the spirogram (mid-flows). Qualitatively, it is reflected by a concave shape on the flow-volume curve. Quantitatively, proportionally increased the reduction in the instantaneous flow measured after 75% of the FVC has been exhaled (FEF) or FEF. Nevertheless, mid-flow abnormalities are not specific to airflow obstruction, and it is no longer a diagnostic criterion of airflow obstruction.
The FEV1 is used to classify the severity of obstructive lung diseases traditionally based on % predicted values into five levels.[14]
- FEV1 >70% of predicted is mild
- FEV1 60-69% of predicted i is moderate
- FEV1 50-59% of predicted is moderately severe
- FEV1 35-49% of predicted is severe
- FEV1<35% of predicted is very severe
However, this method did not factor in age differences; therefore, it was recently changed to something new called z-score (express, which is defined as how far an observed lung function value is from the predicted value after accounting for sex, age, height, and ancestral factors). The utility of the new proposed cut values of −2, −2.5, −3, and −4 severity-scale systems in clinical practice is not clear yet, as most PFT systems continue to use the old method of reporting FEV1.
For many years the reversibility testing has been administered using a bronchodilator (short-acting beta 2-agonist or anticholinergic agent). An increase in either FEV1 or FVC of >12% and >200 mL is considered a positive bronchodilator response. However, the most recent ERS/ATS interpretation standards recommend that a significant bronchodilator response is defined by an increase in FEV1 or FVC by at least 10 percent of the respective predicted values.[1] A lack of response on spirometry post bronchodilators does not predict a lack of clinical response to bronchodilators. In patients with COPD, the bronchodilator response (manifesting by a significant change in FEV or FVC), but the reversal to normal values of FEV1 or FVC on spirometry makes COPD less likely.[15] This contrasts with asthma, as the response to the bronchodilator test can lead to normal spirometry.
The patient should hold their bronchodilators before the reversibility testing. The duration for holding the inhalers differs based on the type of short or long-acting inhaler. Short-acting inhaled beta-agonists (e.g., albuterol) should be held for at least 4 to 6 hours before spirometry. Long-acting inhalers such as tiotropium should be held for 36 to 48 hours before spirometry.
The spirometry procedure includes the measurement of flow volume loops. The maximal flow-volume curves are a great asset for detecting mild airflow obstruction (Figure 1). In each flow-volume loop, there is an inspiratory and expiratory segment.[16] The flow-volume loops (FVL) allow assessment of upper airway obstruction, both variable (e.g., vocal fold paralysis) and fixed types (e.g., tracheal stenosis), and intra- and extrathoracic locations. When the plateau of the FVL occurs in the inspiratory limb, the obstruction is variable and extrathoracic, while when it only occurs in the expiratory FVL limb, the obstruction is variable and intrathoracic. When both limbs are plateaued, then the obstruction is fixed, such as in tracheal stenosis.
Restrictive Abnormalities
Restrictive ventilatory defects are characterized by a normal FEV1/FVC ratio (>0.70) and a reduction in TLC below the fifth percentile or 80% of the predicted value. They can be suspected if the FVC is reduced (less than 80%), FEV1/FVC is increased (0.70), and the flow-volume curve shows a convex shape. Spirometry can only suggest a restrictive defect; lung volumes are necessary to confirm the defect. As mentioned previously, TLC is the gold standard for diagnosing a restrictive defect. The severity of the restrictive defects is based on the FEV1 percentage predicted value, using the same severity scale as obstructive defects.
In patients with kyphoscoliosis, a restrictive ventilatory defect is common on pulmonary function tests. The decline in vital capacity (VC) correlates with the degree of kyphosis. The ventilatory defect manifest by reduced VC and total lung capacity (TLC) with preserved residual volume.
Mixed Abnormalities
These are characterized by obstructive and restrictive defects, diagnosed when both FEV/FVC and TLC are below the five percentile of their predicted values (or FEV1/FVC ratio less than 0.70 and TLC less than 80% predicted value). If FEV1/FVC ratio is low and FVC is less than 80% of predicted, but TLC is normal, the patient has an obstructive pattern, and FVC is low due to hyperinflation. If the TLC is low, the patient has a mixed obstructive and restrictive pattern. For mixed defects, spirometry and lung volumes are needed for diagnosis. The severity of the restrictive defects is also based on the FEV percentage predicted value, using the same severity scale as obstructive defects.[13][4]
Media
References
Stanojevic S, Kaminsky DA, Miller MR, Thompson B, Aliverti A, Barjaktarevic I, Cooper BG, Culver B, Derom E, Hall GL, Hallstrand TS, Leuppi JD, MacIntyre N, McCormack M, Rosenfeld M, Swenson ER. ERS/ATS technical standard on interpretive strategies for routine lung function tests. The European respiratory journal. 2022 Jul:60(1):. pii: 2101499. doi: 10.1183/13993003.01499-2021. Epub 2022 Jul 13 [PubMed PMID: 34949706]
Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J, ATS/ERS Task Force. General considerations for lung function testing. The European respiratory journal. 2005 Jul:26(1):153-61 [PubMed PMID: 15994402]
Level 1 (high-level) evidenceSankari A, Bascom A, Oomman S, Badr MS. Sleep disordered breathing in chronic spinal cord injury. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2014 Jan 15:10(1):65-72. doi: 10.5664/jcsm.3362. Epub 2014 Jan 15 [PubMed PMID: 24426822]
Cooper BG. An update on contraindications for lung function testing. Thorax. 2011 Aug:66(8):714-23. doi: 10.1136/thx.2010.139881. Epub 2010 Jul 29 [PubMed PMID: 20671309]
Ali NA, Nafees AA, Fatmi Z, Azam SI. Dose-response of Cotton Dust Exposure with Lung Function among Textile Workers: MultiTex Study in Karachi, Pakistan. The international journal of occupational and environmental medicine. 2018 Jul:9(3):120-128. doi: 10.15171/ijoem.2018.1191. Epub [PubMed PMID: 29995017]
O'Donnell CR, Bankier AA, Stiebellehner L, Reilly JJ, Brown R, Loring SH. Comparison of plethysmographic and helium dilution lung volumes: which is best for COPD? Chest. 2010 May:137(5):1108-15. doi: 10.1378/chest.09-1504. Epub 2009 Dec 18 [PubMed PMID: 20022972]
Rhodes CE, Denault D, Varacallo MA. Physiology, Oxygen Transport. StatPearls. 2025 Jan:(): [PubMed PMID: 30855920]
Trakada G, Kastritis E, Gavriatopoulou M, Velentza L, Fotiou D, Ziogas DC, Panagiotidis I, Eleutherakis-Papaiakovou E, Roussou M, Migkou M, Kanellias N, Ntanasis-Stathopoulos I, Kallianos A, Terpos E, Dimopoulos MA. Pulmonary function abnormalities are common in patients with multiple myeloma and are independently associated with worse outcome. Annals of hematology. 2019 Jun:98(6):1427-1434. doi: 10.1007/s00277-019-03641-x. Epub 2019 Mar 5 [PubMed PMID: 30834954]
Coates AL, Wanger J, Cockcroft DW, Culver BH, Bronchoprovocation Testing Task Force: Kai-Håkon Carlsen, Diamant Z, Gauvreau G, Hall GL, Hallstrand TS, Horvath I, de Jongh FHC, Joos G, Kaminsky DA, Laube BL, Leuppi JD, Sterk PJ. ERS technical standard on bronchial challenge testing: general considerations and performance of methacholine challenge tests. The European respiratory journal. 2017 May:49(5):. pii: 1601526. doi: 10.1183/13993003.01526-2016. Epub 2017 May 1 [PubMed PMID: 28461290]
Holland AE, Spruit MA, Troosters T, Puhan MA, Pepin V, Saey D, McCormack MC, Carlin BW, Sciurba FC, Pitta F, Wanger J, MacIntyre N, Kaminsky DA, Culver BH, Revill SM, Hernandes NA, Andrianopoulos V, Camillo CA, Mitchell KE, Lee AL, Hill CJ, Singh SJ. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. The European respiratory journal. 2014 Dec:44(6):1428-46. doi: 10.1183/09031936.00150314. Epub 2014 Oct 30 [PubMed PMID: 25359355]
Level 1 (high-level) evidenceBittner V, Weiner DH, Yusuf S, Rogers WJ, McIntyre KM, Bangdiwala SI, Kronenberg MW, Kostis JB, Kohn RM, Guillotte M. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction. SOLVD Investigators. JAMA. 1993 Oct 13:270(14):1702-7 [PubMed PMID: 8411500]
Level 2 (mid-level) evidenceBohannon RW, Crouch R. Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: a systematic review. Journal of evaluation in clinical practice. 2017 Apr:23(2):377-381. doi: 10.1111/jep.12629. Epub 2016 Sep 4 [PubMed PMID: 27592691]
Level 1 (high-level) evidenceMiller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J, ATS/ERS Task Force. Standardisation of spirometry. The European respiratory journal. 2005 Aug:26(2):319-38 [PubMed PMID: 16055882]
Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J. Interpretative strategies for lung function tests. The European respiratory journal. 2005 Nov:26(5):948-68 [PubMed PMID: 16264058]
Tashkin DP, Celli B, Decramer M, Liu D, Burkhart D, Cassino C, Kesten S. Bronchodilator responsiveness in patients with COPD. The European respiratory journal. 2008 Apr:31(4):742-50. doi: 10.1183/09031936.00129607. Epub 2008 Feb 6 [PubMed PMID: 18256071]
Level 1 (high-level) evidencePaine NJ, Joseph MF, Bacon SL, Julien CA, Cartier A, Ditto B, Favreau H, Lavoie KL. Association Between Depression, Lung Function, and Inflammatory Markers in Patients with Asthma and Occupational Asthma. Journal of occupational and environmental medicine. 2019 Jun:61(6):453-460. doi: 10.1097/JOM.0000000000001562. Epub [PubMed PMID: 30855523]