Introduction
Extramammary Paget disease (EMPD) is a rare dermatologic condition that frequently presents in areas where apocrine sweat glands are abundant, most commonly the vulva, although perineal, scrotal, perianal, and penile skin may also be affected. Lesions clinically present as erythematous, well-demarcated plaques that may become erosive, ulcerated, scaly, or eczematous. Extramammary Paget disease has a female predominance and usually occurs in the sixth to eighth decades of life. Professionals disagree about many aspects of EMPD, for example, the prevalence of concurrent vulvar adenocarcinoma or invasive EMPD, association with regional and distant cancers, and recurrence rates following surgical excision. Early recognition is imperative because the diagnosis is frequently delayed and there is a high incidence of associated invasive disease.[1][2][3][4][5]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The etiology of primary EMPD has been debated but appears to be of apocrine origin. Secondary extramammary Paget disease represents an extension of an underlying adnexal adenocarcinoma or an underlying visceral malignancy, most commonly urothelial or anorectal, but it may also be cervical, prostatic, ovarian, or endometrial.
Epidemiology
EMPD is an uncommon condition with only a few hundred cases reported in the world medical literature. Precise incidence is unknown. EMPD represents 6.5% of all cutaneous Paget disease and mainly affects patients between 50 to 80 years of age, with a peak age of 65.3 years old. Caucasians and women are more commonly affected, although a male predominance exists in Asia. The vulva is the most frequently involved site, with 65% of EMPD located in this region. EMPD has also been reported in areas with modified apocrine glands, such as the external auditory canal in association with ceruminous carcinoma, and possibly in the eyelid accompanying carcinoma of Moll's glands. Rare sites of involvement include the buttock, glans penis, knee, thigh, abdomen, umbilicus, lower anterior chest, and scalp.
There is an important association between EMPD and another underlying malignancy in at least 10% to 30% of cases, so a thorough investigation to rule out malignancy is critical.
Pathophysiology
The precise pathogenesis of EMPD is still the subject of controversy. The literature seems to indicate that extramammary Paget disease does not have a uniform histogenesis. Approximately 25% of cases have a primary cutaneous adnexal carcinoma, mostly of apocrine type, but sometimes derived from periurethral, eccrine, perianal, or Bartholin's glands. An additional 10% to 15% of EMPD patients have an internal carcinoma involving the bladder, rectum, cervix, prostate, or urethra, which appears to be of etiological significance. Recently theories have postulated that EMPD is associated with Toker cells, which are present in both mammary and vulvar tissue.
Due to the histologic extension beyond the clinically abnormal area, local recurrence of extramammary Paget disease is relatively common. The prognosis is favorable, except in cases with an underlying visceral or adnexal carcinoma, in which the mortality rate is greater than 50%. Decreased survival is also associated with increased serum CEA levels, tumor invasion level, the presence of nodules in the primary lesion, and lymph node metastases. Spontaneous regression has been described in the literature after partial surgical excision. Metastatic extramammary Paget disease is rather rare, but it may spread to regional lymph nodes and visceral organs.[6][7][8]
Histopathology
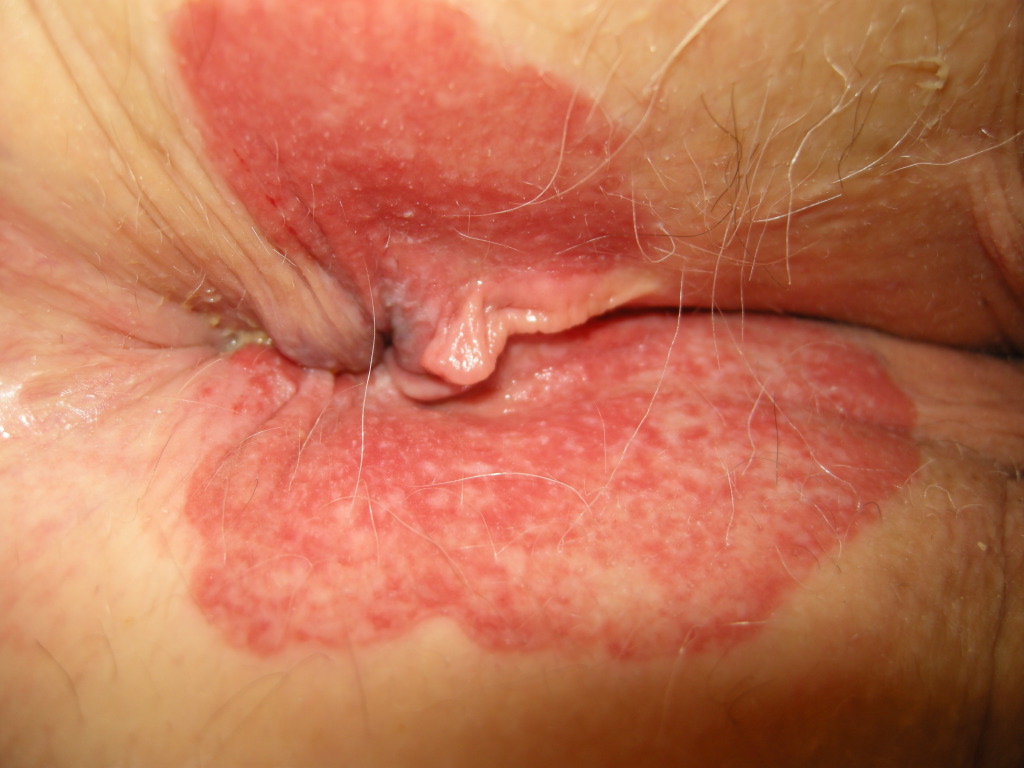
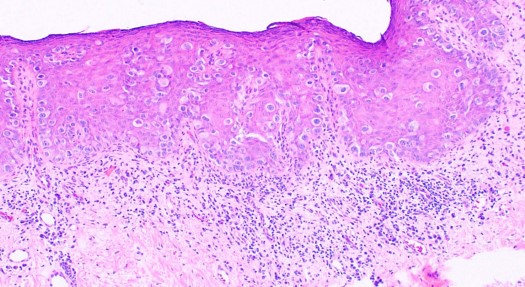
In EMPD, the proliferative neoplastic cell is the Paget cell (PC). These cells have ample pale cytoplasm and large pleomorphic nuclei, sometimes with a prominent nucleolus. Mitoses are commonly present. Occasional cells have an eccentric nucleus and the appearance of a signet ring (see Image. Extramammary Paget Disease).
In early lesions, the cells are arranged singly or in small groups, sometimes with the glandular formation in the basal layer of the epidermis. The entire thickness of the epidermis can be involved, although the greatest concentration of tumor cells is in the lower part of the epidermis. The pagetoid cells usually spread into the contiguous epithelium of eccrine ducts and hair follicles. Uncommonly, Paget cells may invade the dermis.
Unlike the majority of cases of mammary Paget disease, the Paget cells in extramammary Paget disease contain ample mucin, which may be confirmed by staining with Alcian blue, mucicarmine, Periodic acid–Schiff (PAS), and colloidal iron. Immunohistochemistry is often used to diagnose Paget disease and to identify the likely cell of origin. Paget cells usually stain for markers of eccrine and apocrine derivation including low molecular weight cytokeratins (CK), periodic acid-Schiff (PAS), gross cystic disease fluid protein (GCDFP-15), and carcinoembryonic antigen (CEA). S100 staining is negative.
Extramammary Paget disease and mammary Paget disease and show characteristic, although slightly different immunophenotypes. Primary intra-epidermal Paget disease is GCDFP-15 positive, CK7 positive, and CK20 negative. Paget disease that has spread from an underlying internal carcinoma (EMPD) stains GCDFP-15 negative, CK7 negative, and CK20 positive. The main histological differential diagnoses to exclude in the vulva are superficial spreading malignant melanoma (PAS negative, S100 positive, cytokeratin negative, CEA negative) and anogenital intraepithelial neoplasia (S100 negative, PAS negative).
History and Physical
The primary lesions of EMPD are erythematous and scaly plaques, usually well delineated, and measure around several centimeters. One may observe crusting, weepy erosions or even ulcerations at a later stage. Scattered areas of erosion and white scale can give rise to a “strawberries and cream” appearance. The involved areas usually slowly expand, with a sharp demarcation between normal and involved skin. The lesions in EMPD can be nonspecific [commonly misdiagnosed as an inflammatory or infective skin condition (eczema, psoriasis, candidiasis)] and multiple topical therapies are often applied before the diagnosis is made. A median delay of 2 years in proper diagnosis has been reported. The most common presenting symptom in extramammary Paget disease is pruritus, but burning, tenderness, and edema may be experienced as well. Lesions occasionally show hypopigmentation or hyperpigmentation. Long-standing lesions may be modified by repeated trauma/excoriation or superimposed infection.
Hard nodules and regional lymph node enlargement may develop, resulting from the presence of an underlying carcinoma. ‘Underpants-pattern erythema’ is a specific clinical aspect of genital extramammary Paget disease starting in the groin and spreads peripherally to areas covered by underwear. This particular pattern is due to invasion of the lymphatic system by tumor and has an ominous prognosis, since it is associated with rapidly fatal distant metastases.
Evaluation
The diagnosis of EMPD must trigger a laboratory work-up aiming to detect an underlying malignancy. Depending on the area of skin lesions, the following examinations should be performed: cervicovaginal smear, rectocoloscopy, cystoscopy, abdominal ultrasound or computed tomography scanning, gastroduodenal fibroscopy, mammography, intravenous urography, the dosage of serum tumor markers (CEA, CA 19–9, CA 15–3).
A clinical follow-up needs to be long-term as some patients develop recurrences more than 15 years after initial treatment. For non-invasive EMPD, the follow-up could be biannual for 3 years, then annually for 10 years. For invasive EMPD or those associated with a distant tumor, the follow-up should be more frequent (3 or 4 times per year), with biopsy of any suspicious skin lesion. Specifically for perianal EMPD, follow up should involve an annual complete examination, punch biopsy of any new lesion, and proctosigmoidoscopy. These patients should undergo colonoscopy every 2 to 3 years. Vulvar EMPD may be followed up with regular vulvar inspection, punch biopsies to exclude invasive disease in any recurrent lesions, and hysteroscopy/regular pelvic ultrasound scans.
A lymph node staging system was proposed by Hatta and colleagues, where N0 implies no involvement of lymph nodes; N1, unilateral involvement; and N2 bilateral or distant-site disease. The Kaplan-Meier overall survival curve figured in this study depicts decreasing survival with increasing lymph node status. Thus, a clinical predicament arises when faced with managing a patient with histologically proven EMPD and negative clinical lymphadenopathy. This issue has been broached recently in the literature relating to 2 surgical aspects: the value of sentinel lymph node biopsy (SLNB) with or without complete lymph node dissection (CLND) and the pertinence of elective lymph node dissection (ELND) in managing invasive EMPD. The basis from which these procedures stem is centered on the notion that metastases first pass through the lymphatic system before disseminating systemically and that, by interrupting this passage, it may be possible to prevent widespread disease.
Treatment / Management
Surgery is still considered the gold standard of treatment for patients with EMPD. Over the years, many therapeutic modalities have been attempted on patients with EMPD to reduce the significant morbidity associated with the often-radical surgical treatments performed. Because of the rarity of this condition, experience in its management is limited. Due to the high frequency of recurrences after excision, adjunctive therapy is often used. The literature recommends a safety resection margin of 2 cm, although a 1 cm margin could be sufficient for lesions with clinically clear margins. A lower recurrence rate has been reported when utilizing Mohs micrographic surgery (MMS). A study by Coldiron and colleagues evaluated recurrence of EMPD after MMS versus conventional excision using fixed or frozen margins. They combined results from their own experience with those from a written survey of members of the American College of Micrographic Surgery and compared with cases selected from the literature that had undergone conventional surgery. Coldiron et al. reported a recurrence rate of 23% for MMS versus 33% for conventional excision with margin control.
Systemic chemotherapy (vincristine, docetaxel, carboplatin, 5-FU, mitomycin-C, etoposide) can be used if there are contraindications to surgery and radiotherapy. Radiation treatment can be utilized for inoperable lesions or as adjuvant treatment to surgical excision, namely, in the case of postoperative recurrence; the results are better in primary in situ EMPD. Local application of cytotoxic drugs (bleomycin, 5-fluorouracil) alone is not sufficient, but may decrease the margins of the lesions or assist in visualization, rendering resection more efficacious. Intralesional interferon has given promising results in some patients. The topical immune-response modifier, imiquimod, has been successfully used in a small number of EMPD cases it appears to be helpful in superficial forms. Imiquimod can be considered an alternative to surgery, an adjunct before or after surgery, and even part of a therapeutic combination with other treatment modalities. Though only case reports or case series have been published thus far, results seem promising for Imiquimod.
Differential Diagnosis
Diagnosis and definitive treatment are often delayed due to the non-specific clinical findings resulting in misdiagnosis, and elderly patients frequently present late. EMPD is commonly mistaken for: contact dermatitis, fungal infection, psoriasis seborrheic dermatitis, anogenital intraepithelial neoplasia, lichen sclerosis, melanoma, mycosis fungoides, and histiocytosis. Perianal or vulvar EMPD may also be misdiagnosed as leukoplakia, squamous cell carcinoma, basal cell carcinoma, condylomata acuminate, hidradenitis suppurativa, or Crohn’s disease.
Enhancing Healthcare Team Outcomes
The diagnosis and management of extramammary Paget disease is with an interprofessional team that includes an oncologist, pathologist, dermatologist, primary care provider, nurse practitioner, and a surgeon. The condition is very rare and delay in diagnosis are very common. When an itchy or scaly rash is seen that persists, one should always have to to consider a malignant process, especially in older people. While the treatment for localized lesions is surgery, in many patients local spread has occurred by the time diagnosis is made. There is a high rate of recurrence after surgery and in these patients, systemic chemotherapy and/or radiation therapy is often utilized. The prognosis for most patients is guarded.[9]
Once treatment is completed, the should be monitored closely on follow up by their oncologist, nurse practitioner, and an oncology nurse. If there is any evidence of recurrence, the patient should be referred back to the specialist. It is important that the oncology nurse arrange regular followup appointments and educate the family on the need for close monitoring. If there is any concern regarding followup or lack of patient/family awareness, the nurse should involve the oncologist. Only with an interprofessional team will the best outcomes be achieved. [Level V]
Media
(Click Image to Enlarge)
References
Yildiz P, Ronen S, Aung PP, Trinidad C, Kajoian A, Prieto VG. Extramammary Paget Disease-A Challenging Case. The American Journal of dermatopathology. 2019 Nov:41(11):867-868. doi: 10.1097/DAD.0000000000001311. Epub [PubMed PMID: 30807292]
Level 3 (low-level) evidenceNakamura Y, Tanese K, Hirai I, Amagai M, Kawakami Y, Funakoshi T. Serum cytokeratin 19 fragment 21-1 and carcinoembryonic antigen combination assay as a biomarker of tumour progression and treatment response in extramammary Paget disease. The British journal of dermatology. 2019 Sep:181(3):535-543. doi: 10.1111/bjd.17789. Epub 2019 Jun 24 [PubMed PMID: 30791097]
Molina GE, Khalifian S, Mull JL, Chen L, Rosman IS, Faulkner-Jones BE, Ngo KH, Demehri S, Cornelius LA, Wu PA. Topical Combination of Fluorouracil and Calcipotriene as a Palliative Therapy for Refractory Extramammary Paget Disease. JAMA dermatology. 2019 May 1:155(5):599-603. doi: 10.1001/jamadermatol.2018.4793. Epub [PubMed PMID: 30785593]
Jones MA, Edwards T, Ermolovich T. Brown macule on vulva of an elderly woman. JAAD case reports. 2019 Feb:5(2):156-158. doi: 10.1016/j.jdcr.2018.11.020. Epub 2019 Jan 25 [PubMed PMID: 30733985]
Level 3 (low-level) evidenceCervantes J, Rosen A, Cho JH. Enlarging Bump on the Scrotum. Skin appendage disorders. 2018 Nov:5(1):52-55. doi: 10.1159/000488723. Epub 2018 Jun 5 [PubMed PMID: 30643783]
Valle L, Deig C, Wright R, High W. An advanced case of extramammary Paget disease: Safe and effective treatment in an inoperable elderly patient using extensive en face electron irradiation. JAAD case reports. 2019 Jan:5(1):72-74. doi: 10.1016/j.jdcr.2018.08.010. Epub 2018 Dec 9 [PubMed PMID: 30560191]
Level 3 (low-level) evidenceAsel M, LeBoeuf NR. Extramammary Paget's Disease. Hematology/oncology clinics of North America. 2019 Feb:33(1):73-85. doi: 10.1016/j.hoc.2018.09.003. Epub [PubMed PMID: 30497678]
Yao H, Xie M, Fu S, Guo J, Peng Y, Cai Z, Jiang Y, Zheng D, Wang Z. Survival analysis of patients with invasive extramammary Paget disease: implications of anatomic sites. BMC cancer. 2018 Apr 10:18(1):403. doi: 10.1186/s12885-018-4257-1. Epub 2018 Apr 10 [PubMed PMID: 29636019]