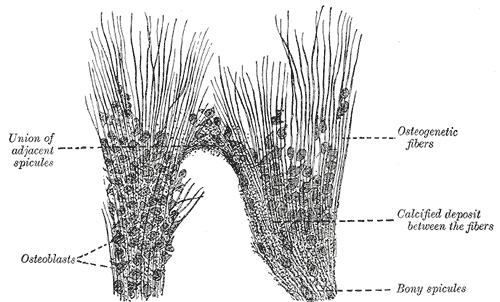
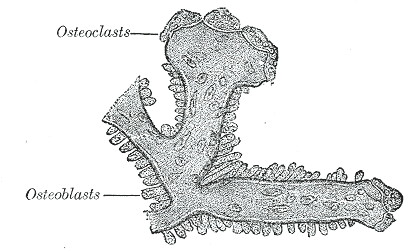
Introduction
Osteoblasts are colloquially referred to as cells that "build" bone. These cells are directly responsible for osteogenesis (or ossification). Osteoblasts synthesize and deposit organic bone matrix (osteoid) proteins that will mineralize in both developing skeletons and during the process of bone remodeling that occurs continuously throughout an individual's life.[1]
Bone is approximately 10% water, 30% organic, and 60% inorganic. The organic component is approximately 85 to 90% collagen (primarily type 1 -resisting tensile forces), proteoglycans (resisting compressive forces), non-collagenous proteins (osteocalcin and osteonectin) and glycoproteins (osteopontin). The inorganic component, or mineralized matrix, is composed of hydroxyapatite crystals [Ca10(PO4)6(OH)2] that provides protection and support while serving as the body's repository for calcium and phosphate.[2][3][4][5] Osteoblasts also indirectly regulate osteoclast formation and bone remodeling by cell-cell contact, paracrine signaling, and cell-bone matrix interaction.[6][7]
Osteoblasts derive from two embryonic populations of mesenchymal stromal cells (or mesenchymal stem cells, MSCs). MSCs originating from the neural ectoderm can directly differentiate into osteoprogenitor cells that will become osteoblasts and form bone through intramembranous ossification (i.e., squamous bones of the calvaria and clavicle). MSCs originating from the paraxial mesoderm differentiate into axial skeleton osteoblasts, while the MSCs of the lateral plate mesoderm form osteoblasts of the appendicular skeleton. The axial and appendicular skeleton develop by endochondral ossification, with these osteoblasts deriving from intermediate perichondral cells or hypertrophic chondrocytes. Both the indirect and direct processes converge at osteoprogenitor cells (or preosteoblasts).[8] Osteoblasts are induced from the osteoprogenitor cells by numerous signals. Understanding of the osteogenic lineage remains incomplete.[9]
Issues of Concern
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Issues of Concern
Bone is a specialized connective tissue consisting of cells and a mineralized extracellular matrix, that is continuously being remodeled through a dynamic process to maintain structural integrity and shape.[10] Under normal physiologic conditions, bone homeostasis is maintained through four distinct cell types: osteoblasts, which form bone; osteoclasts, which resorb bone; bone lining cells (external surface-periosteal cells, internal surface-endosteal cells), which differentiate into osteoblasts; and osteocytes, or osteoblasts sequestered within lacunae that function as mechanosensors and coordinators of the bone remodeling process under the control of both local and systemic factors.[11][12] Imbalance in this tightly coupled process can result in abnormal architecture or function, leading to inadequate, excessive or ectopic calcification and resultant clinical manifestations such as osteoporosis, osteoporosis, or heterotopic ossification.[8][13]
Bone is a very complex and changeable organ, capable of influencing other organic structures and vice versa, as well as the immune system and systemic metabolic balance; the bone is influenced by multiple stimulations, internal (pressure, hydration, metabolism) and external (hormones, growth factors, mechanical pressures). The osteoblast can secrete several molecules in paracrine mode. For example, osteoblast-derived VEGF (vascular endothelial growth factor) can improve bone repair or during the bone development process.
Structure
Osteoblasts are cuboidal or polygonal cells that comprise only 4 to 6% of all bone cells and are predominately situated in matrix boundaries. These cells aggregate along bone surfaces, mostly in the periosteum or endosteum, and demonstrate the morphological characteristics of cells that synthesize an abundance of protein; this includes an extensive rough endoplasmic reticulum (RER), Golgi apparatus, numerous secretory vesicles, and mitochondria.[10][11]
Osteoblasts can secrete enzymes, pro-collagenases, which in contact with the matrix transform into collagenases by the action of osteoclasts; collagenase will be used by osteoclasts to disassemble collagen fibers.
Function
Osteoblasts synthesize and secrete bone matrix to maintain the structural integrity and shape of bone. This process promotes bone formation, remodeling, and healing.[10][14]
Tissue Preparation
Immunohistochemistry (IHC)
Paraffin
A common practice to prepare bone tissue samples for IHC involves embedding decalcified tissue in paraffin. Of note, decalcifying bone changes the overall morphology. Trabecular integrity is lost, and the bone cell environment is typically not the same as native mineralized bone.
Methyl Methacrylate (MMA)
Non-decalcified bone tissue samples can embed in Methyl methacrylate (MMA) to better preserve the gross native bone morphology with inorganic phosphates. Sectioning of bone samples embedded with MMA is challenging, with conventional IHC then performed using either heat-induced antigen retrieval (typically for samples under 10 μm thick) or microwave-based heat-induced retrieval. Both of these processes require a high degree of precision concerning temperature control to prevent the sample from being inadvertently damaged.[15]
Histochemistry and Cytochemistry
Histology
Basic histological methods to identify osteoblasts include visualization of cell characteristics and location. Osteoblasts are cuboidal mononuclear cells located on bone surfaces.
Cytochemistry
Cytochemical methods to identify osteoblasts include toluidine blue stain (also known as tolonium chloride), alkaline phosphatase (ALP) enzymatic stain, immunochemical markers, and fluorescent protein reporters.
Toluidine blue
Toluidine blue is a basic thiazine metachromatic dye used to identify osteoblasts in paraffin sections. The dye selectively stains acidic tissue rich in nucleic acids, and other tissue components (sulfates, carboxylates, and phosphate radicals). Four adjacent labeled cuboidal cells are required to categorize a surface as osteoblast populated.[16]
Alkaline Phosphatase (ALP) Enzymatic Stain
ALP is a membrane-bound metalloenzyme that is instrumental in catalyzing the hydrolysis of phosphate monoesters. Depending on the site of tissue expression, one of four ALP glycoprotein isozymes is present. Specifically, bone contains a heat-labile isozyme that is a tissue non-specific alkaline phosphatase (TNSALP or liver/bone/kidney - L/B/K). Slight variations exist between the L/B/K ALPs from different tissues.[17] ALP is a major enzymatic measure of osteoblastic activity and is more specific in identifying osteoblasts.[18][19] In vitro, ALP activity is expressed early in osteogenic lineage and in embryonic stem cells. However, alone it is not sufficient to designate cells as mature osteoblasts. Mineralization labels are often used, including alizarin complexone (red), calcein (green), or demeclocycline (yellow).[9][20]
Immunochemical Markers
Immunochemical markers proposed to be of utility in identifying osteoblast precursors in either human or non-human models include: osterix, nestin, alpha smooth muscle actin, connective tissue growth factor (CTGF) and paired related homeobox 1 (Prx1). Identifying pre-osteoblasts/osteoblasts include type I collagen, bone sialoprotein (BSP), osteocalcin (Oc), and osteopontin.[9][21]
Fluorescent Protein Reporters
Fluorescent protein reporters have also proven useful and reliable for characterizing pre-osteoblasts (Col3.6) and mature osteoblasts (Col2.3, BSP, and Oc) in in vitro and in vivo applications. More recently, Roeder et al. proposed a novel method for introducing visual transgenes into the osteoblast cell lineage in murine models to explore and identify different stages of osteogenic lineage maturation.[9]
Microscopy, Light
Mature osteoblasts are visible as a single layer of cuboidal or polygonal cells with strongly basophilic cytoplasm, eccentrically located nuclei, extensive RER, and a large Golgi complex (in negative image).[11]
Microscopy, Electron
Due to the architectural complexity of bone, scanning, and transmission electron microscopy are frequently used to examine specimens, which enables the differentiation of mineralized and unmineralized components.
Scanning electron microscopy (SEM)
SEM affords a high spatial resolution, relatively large depth, and wide field of view without significant modification of the sample. SEM is typically used to identify composition and characterize the surfaces of samples. The strength of SEM in the topographical visualization of surfaces stems from the technique of detecting electrons reflected off the sample.[22]
When visualized, periosteal osteoblasts have elongated processes projecting from the cell surface. Both the cell surface and processes have globular structures with a diameter of approximately 0.1 microns. As the periosteal surface is mineralized, the globules coalesce to form nonhomogeneous mineralized spherules.
Endosteal osteoblasts have a similar general appearance, with fewer processes and no globular structures.[23][24]
Transmission electron microscopy (TEM)
TEM primarily provides the characterization of a sample's inner structures. In this imagining method, a beam of electrons is passed through a sample, and the detected contrast from electrons absorbed and scattered converts into an image.[25]
TEM has helped to reveal membrane-bound matrix vesicles within osteoblasts that appear to form through budding cell processes. Secretions from these osteoblast cell processes were observed to have preformed membrane-bound vesicles resembling extracellular matrix vesicles.[23][26]
Pathophysiology
To fully understand the implication of physiologic disturbance of osteoblasts in various disease states, one must have a perfunctory understanding of bone and the often-overlooked nuances of this dynamic skeletal organ.
Overview of Osseous Components
Bone (osseous tissue)
- Hard, dense connective tissue that gives structural support to the body
Cortical (compact) bone
- Dense, outer cortex that supports and protects, the main store of calcium, accounts for 80% of bone mass, formed of osteons.
Cancellous (trabecular) bone
- Porous, highly vascular "spongy" internal tissue with a high surface area to volume ratio. Comprised of trabeculae. 20% bone mass, almost 10x the surface area of cortical bone. Red and yellow bone marrow fills in space between pores
Periosteum
- Covers the external surface of cortical bone and is a dense fibrous sheath containing osteoprogenitor cells. An exception is over articular surfaces, where articular cartilage is found. Periosteal cells are capable of becoming osteoblasts
Endosteum
- Covers innermost cortical bone and is often only one cell thick. The endosteum lines the medullary cavity. This cell layer also contains osteoprogenitor cells that can differentiate into osteoblasts
Osteon (Haversian System)
- The fundamental anatomical and functional unit of cortical bone; cylindrically arranged and typically parallel to the long axis. Area of remodeling in cortical bone
- Consists of concentric lamellae surrounding a central Haversian canal, which permit blood vessels and nerves to travel within and supply the osteon
- Volkmann's canals are perpendicular perforating channels in lamellar bone that permit blood vessels and nerves to reach the Haversian canal from the periosteal and endosteal surface; these also interconnect the osteon canals
Interstitial lamellae
- Remnants of partially resorbed osteons from previous remodeling
Trabecula
- The main anatomical and functional unit of cancellous bone. Aligned towards mechanical load distribution. Bone marrow is contained within the porosities created by the trabeculae
Bone Marrow
- Colloquially divided into "red" bone marrow (Medulla ossium rubra): active myeloid tissue where hemopoietic stem cells produce red blood cells; and "yellow" bone marrow (Medulla ossium flava): hematopoietically inactive, mesenchymal stem cells (stroma) that accumulate lipids
- As individuals age, the red marrow becomes yellow as the fat concentration increases. Yellow marrow can revert to red under physiologic stress requiring hemopoiesis.[27][5][28][29]
Physiology of Remodeling
Remodeling is a process orchestrated by bone multicellular units (BMUs), a term for aggregates of osteoblasts and osteoclasts that function sequentially to remodel bone. Estimates are that 1 million BMUs are active at any time. BMUs consist of a cutting cone of osteoclasts that resorb bone, and osteoblasts that subsequently fill in the resorption area. The process predominately divides into four phases: activation, resorption, reversal, and formation.
- Activation recruits osteoclasts
- Resorption osteoclasts "catabolize" or resorb bone
- In the reversal stage, osteoclasts undergo apoptosis and osteoblasts are recruited
- Osteoblasts then secrete a matrix in the Formation stage that mineralizes [30]
Location of Remodeling
Eighty percent of estimated bone remodeling activity has been demonstrated on cancellous bone surfaces. Cortical bone demonstrates intracortical remodeling in addition to remodeling on the periosteal and endocortical surfaces.[31]
Signaling
While not completely understood, bone and bone-forming cells exhibit many complex signaling mechanisms.
MSCs initially differentiate into osteoprogenitor cells, triggered by core-binding factor alpha-1 (CBFA1) or runt-related transcription factor 2 (RUNX2).[32][33][34] With RUNX2 activated, the cells become osteoprogenitor (or preosteoblast) cells. Under the influence of bone morphogenic proteins (BMPs), insulin-like growth factor-1, -2 (IL-1, IL-2), Osterix, as well as other growth factors, the osteoprogenitor cells become osteoblasts.[33][35][36][37][38]
Mature osteoblasts also produce receptor activator of nuclear factor-κB ligand (RANKL), osteoprotegerin (OPG), and macrophage colony-stimulating factor (M-CSF) which regulate further osteoblast differentiation into osteoclasts.[39][40]
Several signaling pathways that are imperative to maintaining a balance between osteoblast and osteoclasts include WNT, BMP, PTH/PTHrP, Notch, and Hedgehog.[41]
Furthermore, growth factors, and anabolic hormones (including fibroblast growth factor, insulin-like growth factor, interleukin-6, parathyroid hormone, estrogen, and calcitonin) exhibit anti-apoptotic effects on osteoblasts. Tumor necrosis factor, glucocorticoids, and bone morphogenic protein 2 induce apoptosis in osteoblasts.[10]
WNT/β-Catenin (Canonical WNT) Pathway
The cell-membrane Frizzled receptor and co-receptor low-density lipoprotein receptor-related protein 5 (LRP5) are inactive in the absence of Wnt ligands. Without Wnt, β-catenin is phosphorylated by glycogen synthase kinase-3 (GSK-3), signaling it for proteolysis by ubiquitin-dependent proteases.
The Wnt pathway is activated when Wnt binds Frizzled and LRP5. GSK-3 downregulates. Inactivation of GSK-3 increases the accumulation of intracellular β-catenin. β-catenin subsequently translocates to the nucleus and induces gene transcription that results in an increase in bone mass through a variety of different mechanisms.; this includes stem cell renewal, preosteoblast replication, osteoblastogenesis, and inhibiting osteoblast apoptosis. Other secreted inhibitors, such as Dickkopf (Dkk) and Sclerostin (SOST), also can regulate signaling through the Wnt/Frizzled/LRP5 interaction.[30][41][42][43][44]
Sclerostin, for example, is secreted from terminally differentiated osteoblasts (osteocytes) embedded within the newly formed bone matrix. Sclerostin binds to and inhibits LRP5 from binding to frizzled receptors. This process activates the Wnt pathway and increases bone production.[41][43]
Parathyroid Hormone (PTH) & Parathyroid Hormone Related Peptide (PTHrP) Pathways
PTH and PTHrP are distinct polypeptides that serve separate biological functions, though functioning through a common receptor -the family B G-protein-coupled-receptor parathyroid hormone-1 receptor (PTH1R). PTH targets osteoblasts and renal tubular cells while PTHrP targets chondrocytes, osteoblasts, placental cells, skin, hair follicles, brain, and teeth. Notably, PTH functions to maintain calcium and phosphate homeostasis while PTHrP is involved in the development of the placenta, fetus, and bone.[45]
PTH stimulates both resorption and formation of bone depending on the temporality of its exposure. Continuous elevation results in resorption, while intermittent elevation leads to osteoblast formation. Both effects appear to result from the modulation of PTH1R. Resorption may result from increased RANKL synthesis and inhibiting OPG mRNA expression, while the PTH induced mechanism for bone formation has not been completely elucidated.[46][47]
There have been suggestions that intermittent elevation of PTH results in the release of TGF-B from resorbing bone that, in the absence of stimulation, serves to recruit osteogenic progenitors. PTH is also an upstream regulator of Runx2. The cell cycle effect of PTH on Runx2 appears to be mediated through MAP kinase ERK1/2 phosphorylation, activation of CREB/fos with JunD, resulting in the expression of IL-11 and suppression of Dkk as well as cyclin D1 activity to increase bone formation.[8][45]
PTH stimulates osteoblasts to produce angiopoietin 1, a vascular growth factor as well as WNT co-receptor LRP6 and activates WNT signaling.[47] PTH also reduces SOST levels in bone, as does skeletal loading.[46]
Sodium-Hydrogen exchanger regulatory factor (NHERF) 1 also has been implicated in modulating PTH signaling.[48] The mechanisms, as mentioned earlier, could serve to contribute to skeletal manifestations of individuals with kidney or parathyroid disorders.[49][50][51]
Clinical Significance
Osteoblasts are a significant contributor to the physiological homeostasis of bone, and researchers have postulated their dysregulation as a potential factor in a broad spectrum of pathologic conditions, particularly conditions affecting the structural integrity of bone. Understanding osteoblasts requires a comparable concurrent cognizance of osteoclasts, their oppugnant cell, and the interactions between the cell types.[21] This topic has volumes of publications comprising numerous scientific disciplines and can be extrapolated to many conditions. Select, frequently encountered clinical conditions and biomarkers follow below to provide an overview, in no way representative of the true importance of osteoblasts.
Osteoblast Markers
Many serum biomarkers of osteoblast function exist. These include osteoprotegerin (OPG), receptor activator of nuclear factor kappa B ligand (RANKL), alkaline phosphatase (ALP), bone alkaline phosphatase (B-ALP), osteocalcin, procollagen type 1 carboxy-terminal propeptide (P1CP) and procollagen type 1 amino-terminal propeptide (P1NP).[21][52] Seldomly are the majority of these markers utilized clinically to measure osteoblast function due to cost, analytical inconsistencies, and undetermined clinical significance.[53][54][55] Osteocalcin is the most specific marker for osteoblastic activity.[38]
Alkaline Phosphatase
Serum alkaline phosphatases (ALPs) are probably the most common clinically encountered marker for osteoblastic activity. ALPs classify as either tissue-specific (intestine, placenta, and germinal tissue) or tissue-nonspecific (live, bone, and kidney). The tissue nonspecific ALPs are encoded by a single gene and are isoenzymes located on cell membranes that catalyze the hydrolysis of organic phosphate esters.[56] A comprehensive metabolic panel (CMP) frequently measures tissue nonspecific ALPs.
The clinical value of ALP, as a marker for osteoblastic activity, on CMP is dictated by clinical context. While the most commonly encountered elevation of serum ALP is cholestatic liver disease, osteoblastic diseases of bone are also associated with increased serum ALP -such as metastatic adenocarcinoma of the prostate, osteogenic osteosarcoma, and early multiple myeloma.[56][57][58][59][60] Patients with healing fractures and metabolic bone disease (including rickets, osteomalacia, osteopetrosis, or Paget's Disease of bone) can also have an elevated ALP.[57][61][62] It is important to note that osteoclastic processes will not directly affect ALP.
Decreased levels of serum alkaline phosphatase are observable in conditions affecting osteoblastic activity such as hypophosphatasia and cleidocranial dysplasia.[63][64]
Alkaline phosphatase immunohistochemistry is also utilized in pathology to demonstrate osteoblastic activity. ALP IHC staining contributes to aid in diagnosing osteosarcoma and other primary bone tumors, such as giant cell tumor of bone.[65][66]
Development & Peak Bone Mass
Osseous development follows a predictable, stepwise progression of formation, beginning in the sixth to seventh week of embryonic development until adulthood.[67] Alkaline phosphatase is commonly elevated during these periods of bone growth and has a strong correlation to conventional bone maturity factors.[68][69][56] During pubertal maturation, bone size increases while volumetric density remains relatively constant. Bone mass and strength, or peak bone mass, is achieved at the end of the growth process with the closure of all ossification centers.[70] The exact age when individuals achieve peak bone mass (PBM) is a point of dispute in the literature. Consensus data from most studies estimate that PBM is achieved between an individual's early twenties and thirties. Bone mass accumulation varies not only between males and females but also between races as well. Non-modifiable factors, such as genetics, and modifiable risk factors, including nutrition, physical activity, and smoking status, also account for this variation.[71] Even following the attainment of peak bone mass, bone continues to undergo remodeling. Bone mass will remain stable for up to two decades.[30]
Patients need to understand that bone is a living organ, continually remodeling. The importance of remodeling includes replacing damaged bone to maintain mechanical strength and calcium homeostasis. Wolff's law states that bone adapts and remodels as a response to stress from mechanical forces, changing its architecture.[72] Wolff's law also has implications in a maladaptive response that contributes to periarticular bone formation in association with osteoarthritis.[73] This process has been hypothesized as the mechanism forming osteophytes.
Aging
As humans age, various factors such as reduced mechanical loading and endocrine dysfunction contribute to an imbalance between bone formation and bone resorption. This dysfunction results in an alteration of bone quantity and quality. The importance of cortical remodeling also increases as cancellous bone is lost; remodeling in both compartments increases.
Waning vitamin D production and decreasing calcium absorption exacerbate the incongruity between preserving bone strength and providing the body with calcium, which manifests as secondary hyperparathyroidism, maintaining serum calcium levels at the expense of bone resorption.
Postmenopausal women and older men are particularly at-risk populations. The balance between resorption and formation becomes negative, leading to irreversible trabecular thinning and loss, as well as cortical thinning.[30][74][75]
Osteoporosis
Osteoporosis is the most common disease of bone and a major public health concern. Osteoporotic bone exhibits decreased mass, deteriorated tissue, and disrupted architecture that results in compromised strength.[76][77][78] In short, resorption becomes greater than formation.
The diagnosis is by measuring bone mineral density (BMD) with a dual-energy X-ray absorptiometry (DEXA) scan or after a vertebral or hip fragility fracture in the absence of significant trauma. Two methods serve to calculate BMD.
- T-score:
- Comparison to healthy, young, sex-matched individuals
- A score of less than -2.5 standard deviations (SDs) below average defines osteoporosis, as defined by the WHO [79]
- Z-score:
- Comparison to persons of same age and sex
- A score of less than -2.5 SDs is suspicious for a secondary cause of osteoporosis
Osteoporosis divides into two types:
- Primary Osteoporosis
- Postmenopausal (involutional osteoporosis Type I): deficiency of estrogen, primarily affecting trabecular bone
- Senile (involutional osteoporosis Type II): decrease bone mass secondary to the aging of both cortical and trabecular bone
- Secondary Osteoporosis
- Due to an extensive array of factors including but not limited to: lifestyle, genetics, autoimmune disease, rheumatologic disease, endocrine, gastrointestinal, hematological, or neurological disorder.[76][80][81] Endocrine disorders are the most common cause of secondary osteopenia in men and women.[82]
Estrogen insufficiency especially predisposes postmenopausal women and older men to osteoporosis through a variety of mechanisms, including increasing osteocyte apoptosis. While incompletely understood, this has been postulated to be partially due to dysregulation between receptor activator of nuclear factor-κB ligand (RANKL), receptor activator of nuclear factor-κB (RANK) and osteoprotegerin (OPG), a decoy receptor the neutralizes RANKL. The RANK/RANKL/OPG pathway is pivotal in osteoclast activation, and the dysregulation between osteoclastic and osteoblastic activity partially stems from the biological effect of estrogen on this mechanism.
Specifically, estrogen has demonstrated to modulate RANKL, a membrane-bound protein secreted by osteoblasts, critical for osteoclast differentiation (through its binding to RANK), activation, and survival. Estrogen also upregulates OPG expression, which inhibits osteoclastogenesis. RANK and M-CSF, a factor that increases bone resorption, have been demonstrated to be downregulated by estrogen. Estrogen directly protects bone by preventing: osteocyte apoptosis, osteoclast survival (through TGF-B), and osteoblast apoptosis (through the Fas ligand). This is in opposition to 1,25(OH)2D3, PTH/PGE2, and interleukin (IL)-11, which induce RANKL expression.[83][84][85][86][87][88][89]
The North American Menopause Society recommends that all postmenopausal women, regardless of BMD, observe the following recommendations [79][90]:
- Recommended daily allowance calcium (female)
- 1,000 mg/d - age 19 to 50
- 1,200 mg/d - 50+
- Recommended daily allowance calcium (male)
- 1,000 mg/d - age 19 to 70
- 1,200 mg/d - 70+
- Recommended daily allowance vitamin D (male and female)
- 600 IU/d - age 19 to 70
- 800 IU/d - 70+
Commonly prescribed medications for osteoporotic bone disease includes recombinant parathyroid hormone [PTH(1-34)] to stimulate osteoblasts and antiresorptive agents.[79][91][92][93][94][79][95][96]
Osteoarthritis
Recent evidence from Maruotti et al. suggests that while the pathogenesis of osteoarthritis (OA) is still poorly understood, osteoblastic dysregulation could play a substantial role. Abnormal expression of OPG and RANKL has been observed in osteoblasts from patients with osteoarthritic changes. Various transcription factors, growth factors, PGE2, and IL-6, have also been demonstrated to be produced in aberrant quantities by osteoarthritis osteoblasts, possibly contributing to the pathogenesis of OA.[97]
PTHrP and Malignancy
PTHrP is a known mediator of malignancy-induced hypercalcemia (or humoral hypercalcemia of malignancy - HHM).[98] The substantial, sustained exposure of PTHrP has been proposed to stimulate bone metastasis and increase RANKL synthesis, subsequently contributing to osteolytic lesions.[99][100][101][102][103] The atmospherics of the mechanism through which PTHrP exerts its devastating biochemical influence in this context are functionally commensurate to that of PTH on PTH1R.[104][105][106]
Medications
Medications such as calcitonin, recombinant PTH, selective estrogen receptor modulators, and human monoclonal antibodies that bind RANKL can either directly or indirectly modulate osteoblast function. These medications are frequently used in the management of osteoporosis and other conditions.[76][107][108]
Glucocorticosteroids
Glucocorticoid excess has been established to decrease bone mineral density by reducing bone formation and increasing resorption. Glucocorticoids inhibit osteoblastogenesis, increase osteoblast apoptosis, and decrease osteoclast apoptosis.[109][110]
Media
(Click Image to Enlarge)
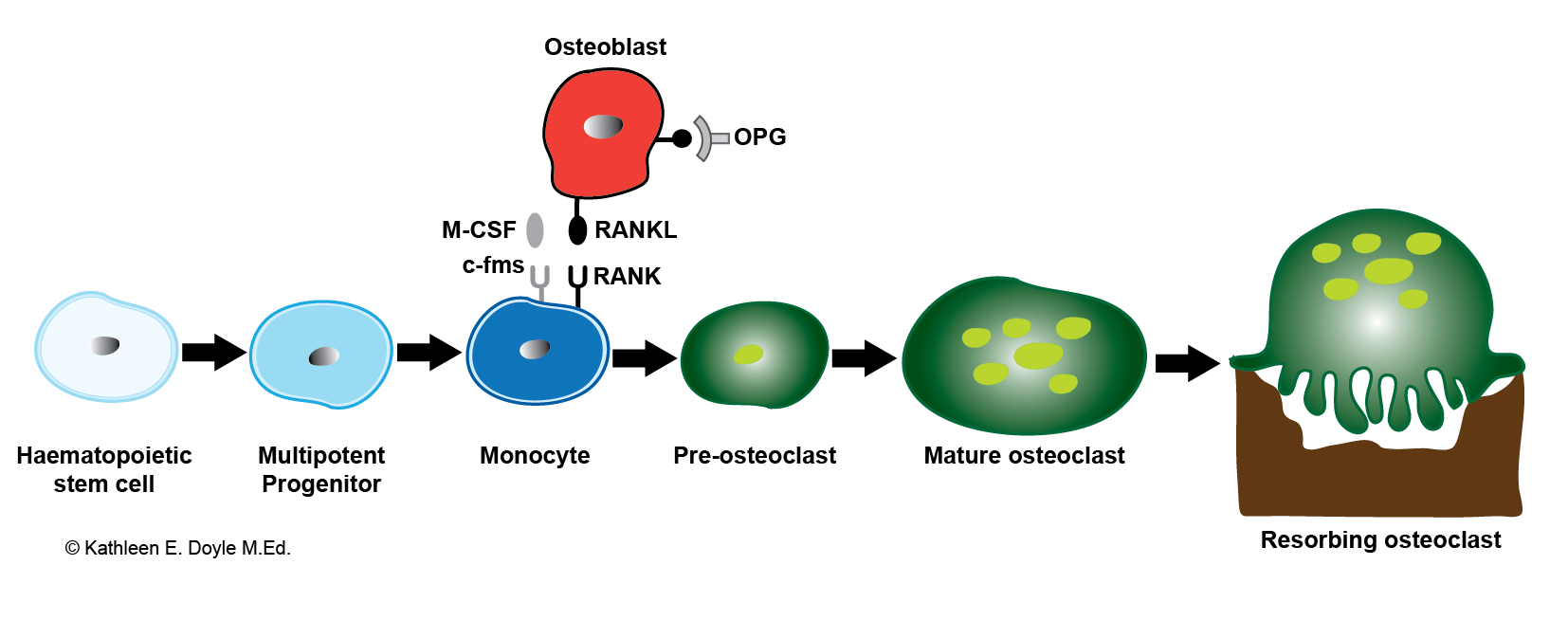
Osteoclastogenesis. Bone-resorbing osteoclasts originate from hemopoietic cells of the monocyte–macrophage lineage under the control of bone-forming osteoblasts. Illustration includes RANKL, the receptor activator of NF-κB ligand; M-CSF, macrophage colony-stimulating factor; and OPG, osteoprotegerin.
Contributed by KE Doyle, MEd
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Tanaka Y, Nakayamada S, Okada Y. Osteoblasts and osteoclasts in bone remodeling and inflammation. Current drug targets. Inflammation and allergy. 2005 Jun:4(3):325-8 [PubMed PMID: 16101541]
Level 3 (low-level) evidenceMorello R. Osteogenesis imperfecta and therapeutics. Matrix biology : journal of the International Society for Matrix Biology. 2018 Oct:71-72():294-312. doi: 10.1016/j.matbio.2018.03.010. Epub 2018 Mar 11 [PubMed PMID: 29540309]
Feng X. Chemical and Biochemical Basis of Cell-Bone Matrix Interaction in Health and Disease. Current chemical biology. 2009 May 1:3(2):189-196 [PubMed PMID: 20161446]
Bou Assaf R, Zibara K, Fayyad-Kazan M, Al-Nemer F, Cordahi M, Khairallah S, Badran B, Berbéri A. Healing of Bone Defects in Pig's Femur Using Mesenchymal Cells Originated from the Sinus Membrane with Different Scaffolds. Stem cells international. 2019:2019():4185942. doi: 10.1155/2019/4185942. Epub 2019 Sep 30 [PubMed PMID: 31662765]
Le BQ, Nurcombe V, Cool SM, van Blitterswijk CA, de Boer J, LaPointe VLS. The Components of Bone and What They Can Teach Us about Regeneration. Materials (Basel, Switzerland). 2017 Dec 22:11(1):. doi: 10.3390/ma11010014. Epub 2017 Dec 22 [PubMed PMID: 29271933]
Ottewell PD. The role of osteoblasts in bone metastasis. Journal of bone oncology. 2016 Sep:5(3):124-127 [PubMed PMID: 27761372]
Matsuo K, Irie N. Osteoclast-osteoblast communication. Archives of biochemistry and biophysics. 2008 May 15:473(2):201-9. doi: 10.1016/j.abb.2008.03.027. Epub 2008 Mar 29 [PubMed PMID: 18406338]
Level 3 (low-level) evidenceRutkovskiy A, Stensløkken KO, Vaage IJ. Osteoblast Differentiation at a Glance. Medical science monitor basic research. 2016 Sep 26:22():95-106 [PubMed PMID: 27667570]
Roeder E, Matthews BG, Kalajzic I. Visual reporters for study of the osteoblast lineage. Bone. 2016 Nov:92():189-195. doi: 10.1016/j.bone.2016.09.004. Epub 2016 Sep 8 [PubMed PMID: 27616604]
Rosenberg N, Rosenberg O, Soudry M. Osteoblasts in bone physiology-mini review. Rambam Maimonides medical journal. 2012 Apr:3(2):e0013. doi: 10.5041/RMMJ.10080. Epub 2012 Apr 30 [PubMed PMID: 23908837]
Florencio-Silva R, Sasso GR, Sasso-Cerri E, Simões MJ, Cerri PS. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed research international. 2015:2015():421746. doi: 10.1155/2015/421746. Epub 2015 Jul 13 [PubMed PMID: 26247020]
Marie PJ, Cohen-Solal M. The Expanding Life and Functions of Osteogenic Cells: From Simple Bone-Making Cells to Multifunctional Cells and Beyond. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2018 Feb:33(2):199-210. doi: 10.1002/jbmr.3356. Epub 2018 Jan 16 [PubMed PMID: 29206311]
Chen X, Wang Z, Duan N, Zhu G, Schwarz EM, Xie C. Osteoblast-osteoclast interactions. Connective tissue research. 2018 Mar:59(2):99-107. doi: 10.1080/03008207.2017.1290085. Epub 2017 Mar 21 [PubMed PMID: 28324674]
Czekanska EM, Stoddart MJ, Richards RG, Hayes JS. In search of an osteoblast cell model for in vitro research. European cells & materials. 2012 Jul 9:24():1-17 [PubMed PMID: 22777949]
Level 3 (low-level) evidenceAkkiraju H, Bonor J, Nohe A. An Improved Immunostaining and Imaging Methodology to Determine Cell and Protein Distributions within the Bone Environment. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2016 Mar:64(3):168-78. doi: 10.1369/0022155415626765. Epub 2015 Dec 30 [PubMed PMID: 26718242]
Sridharan G, Shankar AA. Toluidine blue: A review of its chemistry and clinical utility. Journal of oral and maxillofacial pathology : JOMFP. 2012 May:16(2):251-5. doi: 10.4103/0973-029X.99081. Epub [PubMed PMID: 22923899]
Sharma U, Pal D, Prasad R. Alkaline phosphatase: an overview. Indian journal of clinical biochemistry : IJCB. 2014 Jul:29(3):269-78. doi: 10.1007/s12291-013-0408-y. Epub 2013 Nov 26 [PubMed PMID: 24966474]
Level 3 (low-level) evidenceCosby CN, Troiano NW, Kacena MA. The Effects of Storage Conditions on the Preservation of Enzymatic Activity in Bone. Journal of histotechnology. 2008 Dec:31(4):169-173 [PubMed PMID: 20686670]
Barger A, Graca R, Bailey K, Messick J, de Lorimier LP, Fan T, Hoffmann W. Use of alkaline phosphatase staining to differentiate canine osteosarcoma from other vimentin-positive tumors. Veterinary pathology. 2005 Mar:42(2):161-5 [PubMed PMID: 15753469]
Level 3 (low-level) evidenceLee JM, Kim MG, Byun JH, Kim GC, Ro JH, Hwang DS, Choi BB, Park GC, Kim UK. The effect of biomechanical stimulation on osteoblast differentiation of human jaw periosteum-derived stem cells. Maxillofacial plastic and reconstructive surgery. 2017 Dec:39(1):7. doi: 10.1186/s40902-017-0104-6. Epub 2017 Mar 5 [PubMed PMID: 28303237]
Rowe P, Koller A, Sharma S. Physiology, Bone Remodeling. StatPearls. 2024 Jan:(): [PubMed PMID: 29763038]
Fischer ER, Hansen BT, Nair V, Hoyt FH, Dorward DW. Scanning electron microscopy. Current protocols in microbiology. 2012 May:Chapter 2():Unit 2B.2.. doi: 10.1002/9780471729259.mc02b02s25. Epub [PubMed PMID: 22549162]
Ornoy A, Atkin I, Levy J. Ultrastructural studies on the origin and structure of matrix vesicles in bone of young rats. Acta anatomica. 1980:106(4):450-61 [PubMed PMID: 7386166]
Level 3 (low-level) evidenceShah FA, Ruscsák K, Palmquist A. 50 years of scanning electron microscopy of bone-a comprehensive overview of the important discoveries made and insights gained into bone material properties in health, disease, and taphonomy. Bone research. 2019:7():15. doi: 10.1038/s41413-019-0053-z. Epub 2019 May 22 [PubMed PMID: 31123620]
Level 3 (low-level) evidenceWiney M, Meehl JB, O'Toole ET, Giddings TH Jr. Conventional transmission electron microscopy. Molecular biology of the cell. 2014 Feb:25(3):319-23. doi: 10.1091/mbc.E12-12-0863. Epub [PubMed PMID: 24482357]
Level 3 (low-level) evidenceBottini M, Mebarek S, Anderson KL, Strzelecka-Kiliszek A, Bozycki L, Simão AMS, Bolean M, Ciancaglini P, Pikula JB, Pikula S, Magne D, Volkmann N, Hanein D, Millán JL, Buchet R. Matrix vesicles from chondrocytes and osteoblasts: Their biogenesis, properties, functions and biomimetic models. Biochimica et biophysica acta. General subjects. 2018 Mar:1862(3):532-546. doi: 10.1016/j.bbagen.2017.11.005. Epub 2017 Nov 3 [PubMed PMID: 29108957]
Clarke B. Normal bone anatomy and physiology. Clinical journal of the American Society of Nephrology : CJASN. 2008 Nov:3 Suppl 3(Suppl 3):S131-9. doi: 10.2215/CJN.04151206. Epub [PubMed PMID: 18988698]
Małkiewicz A, Dziedzic M. Bone marrow reconversion - imaging of physiological changes in bone marrow. Polish journal of radiology. 2012 Oct:77(4):45-50 [PubMed PMID: 23269936]
Gurevitch O, Slavin S, Feldman AG. Conversion of red bone marrow into yellow - Cause and mechanisms. Medical hypotheses. 2007:69(3):531-6 [PubMed PMID: 17433565]
Level 3 (low-level) evidenceLangdahl B, Ferrari S, Dempster DW. Bone modeling and remodeling: potential as therapeutic targets for the treatment of osteoporosis. Therapeutic advances in musculoskeletal disease. 2016 Dec:8(6):225-235. doi: 10.1177/1759720X16670154. Epub 2016 Oct 5 [PubMed PMID: 28255336]
Level 3 (low-level) evidenceDelgado-Calle J, Bellido T. Osteocytes and Skeletal Pathophysiology. Current molecular biology reports. 2015 Dec:1(4):157-167 [PubMed PMID: 26693137]
Caetano-Lopes J, Canhão H, Fonseca JE. Osteoblasts and bone formation. Acta reumatologica portuguesa. 2007 Apr-Jun:32(2):103-10 [PubMed PMID: 17572649]
Sinha KM, Zhou X. Genetic and molecular control of osterix in skeletal formation. Journal of cellular biochemistry. 2013 May:114(5):975-84. doi: 10.1002/jcb.24439. Epub [PubMed PMID: 23225263]
Level 3 (low-level) evidenceDucy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997 May 30:89(5):747-54 [PubMed PMID: 9182762]
Level 3 (low-level) evidenceKarsenty G. The genetic transformation of bone biology. Genes & development. 1999 Dec 1:13(23):3037-51 [PubMed PMID: 10601030]
Level 3 (low-level) evidenceJia D, Heersche JN. Insulin-like growth factor-1 and -2 stimulate osteoprogenitor proliferation and differentiation and adipocyte formation in cell populations derived from adult rat bone. Bone. 2000 Dec:27(6):785-94 [PubMed PMID: 11113389]
Level 3 (low-level) evidenceBellido T, Borba VZ, Roberson P, Manolagas SC. Activation of the Janus kinase/STAT (signal transducer and activator of transcription) signal transduction pathway by interleukin-6-type cytokines promotes osteoblast differentiation. Endocrinology. 1997 Sep:138(9):3666-76 [PubMed PMID: 9275051]
Level 3 (low-level) evidenceHuang W, Yang S, Shao J, Li YP. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Frontiers in bioscience : a journal and virtual library. 2007 May 1:12():3068-92 [PubMed PMID: 17485283]
Level 3 (low-level) evidenceYamashita T, Takahashi N, Udagawa N. New roles of osteoblasts involved in osteoclast differentiation. World journal of orthopedics. 2012 Nov 18:3(11):175-81. doi: 10.5312/wjo.v3.i11.175. Epub [PubMed PMID: 23330072]
Feng X, Teitelbaum SL. Osteoclasts: New Insights. Bone research. 2013 Mar:1(1):11-26. doi: 10.4248/BR201301003. Epub 2013 Mar 29 [PubMed PMID: 26273491]
Zhong Z, Ethen NJ, Williams BO. WNT signaling in bone development and homeostasis. Wiley interdisciplinary reviews. Developmental biology. 2014 Nov-Dec:3(6):489-500. doi: 10.1002/wdev.159. Epub 2014 Sep 30 [PubMed PMID: 25270716]
Level 3 (low-level) evidenceJoiner DM, Ke J, Zhong Z, Xu HE, Williams BO. LRP5 and LRP6 in development and disease. Trends in endocrinology and metabolism: TEM. 2013 Jan:24(1):31-9. doi: 10.1016/j.tem.2012.10.003. Epub [PubMed PMID: 23245947]
Level 3 (low-level) evidenceKrishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. The Journal of clinical investigation. 2006 May:116(5):1202-9 [PubMed PMID: 16670761]
Level 3 (low-level) evidenceMacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Developmental cell. 2009 Jul:17(1):9-26. doi: 10.1016/j.devcel.2009.06.016. Epub [PubMed PMID: 19619488]
Level 3 (low-level) evidenceDatta NS, Abou-Samra AB. PTH and PTHrP signaling in osteoblasts. Cellular signalling. 2009 Aug:21(8):1245-54. doi: 10.1016/j.cellsig.2009.02.012. Epub 2009 Feb 26 [PubMed PMID: 19249350]
Level 3 (low-level) evidenceWein MN. Parathyroid Hormone Signaling in Osteocytes. JBMR plus. 2018 Jan:2(1):22-30. doi: 10.1002/jbm4.10021. Epub 2017 Nov 10 [PubMed PMID: 30283888]
Jilka RL, O'Brien CA, Bartell SM, Weinstein RS, Manolagas SC. Continuous elevation of PTH increases the number of osteoblasts via both osteoclast-dependent and -independent mechanisms. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010 Nov:25(11):2427-37. doi: 10.1002/jbmr.145. Epub [PubMed PMID: 20533302]
Level 3 (low-level) evidenceLee M, Partridge NC. Parathyroid hormone signaling in bone and kidney. Current opinion in nephrology and hypertension. 2009 Jul:18(4):298-302. doi: 10.1097/MNH.0b013e32832c2264. Epub [PubMed PMID: 19395963]
Level 3 (low-level) evidenceDamasiewicz MJ, Nickolas TL. Rethinking Bone Disease in Kidney Disease. JBMR plus. 2018 Nov:2(6):309-322. doi: 10.1002/jbm4.10117. Epub 2018 Nov 15 [PubMed PMID: 30460334]
Miller PD, Chronic kidney disease and the skeleton. Bone research. 2014; [PubMed PMID: 26273531]
Bandeira F, Cusano NE, Silva BC, Cassibba S, Almeida CB, Machado VC, Bilezikian JP. Bone disease in primary hyperparathyroidism. Arquivos brasileiros de endocrinologia e metabologia. 2014 Jul:58(5):553-61 [PubMed PMID: 25166047]
Wheater G, Elshahaly M, Tuck SP, Datta HK, van Laar JM. The clinical utility of bone marker measurements in osteoporosis. Journal of translational medicine. 2013 Aug 29:11():201. doi: 10.1186/1479-5876-11-201. Epub 2013 Aug 29 [PubMed PMID: 23984630]
Rogers A, Eastell R. Circulating osteoprotegerin and receptor activator for nuclear factor kappaB ligand: clinical utility in metabolic bone disease assessment. The Journal of clinical endocrinology and metabolism. 2005 Nov:90(11):6323-31 [PubMed PMID: 16105967]
Bhattoa HP. Laboratory aspects and clinical utility of bone turnover markers. EJIFCC. 2018 Jul:29(2):117-128 [PubMed PMID: 30050395]
Camozzi V, Tossi A, Simoni E, Pagani F, Francucci CM, Moro L. Role of biochemical markers of bone remodeling in clinical practice. Journal of endocrinological investigation. 2007:30(6 Suppl):13-7 [PubMed PMID: 17721068]
Lowe D, Sanvictores T, Zubair M, John S. Alkaline Phosphatase. StatPearls. 2024 Jan:(): [PubMed PMID: 29083622]
Walker HK, Hall WD, Hurst JW, Vroon DH, Israili Z. Alkaline Phosphatase and Gamma Glutamyltransferase. Clinical Methods: The History, Physical, and Laboratory Examinations. 1990:(): [PubMed PMID: 21250047]
Bataille R, Chappard D, Marcelli C, Dessauw P, Baldet P, Sany J, Alexandre C. Recruitment of new osteoblasts and osteoclasts is the earliest critical event in the pathogenesis of human multiple myeloma. The Journal of clinical investigation. 1991 Jul:88(1):62-6 [PubMed PMID: 2056131]
Siddique A, Kowdley KV. Approach to a patient with elevated serum alkaline phosphatase. Clinics in liver disease. 2012 May:16(2):199-229. doi: 10.1016/j.cld.2012.03.012. Epub 2012 Apr 6 [PubMed PMID: 22541695]
Level 3 (low-level) evidenceFu R, Peng F, Liu H, Wang Y, Li L, Wang G, Song J, Shao Z. Clinical significance of osteoblast precursors and osteoclast precursors in earlier diagnosis and monitoring of myeloma bone disease. Annals of hematology. 2016 Jun:95(7):1099-106. doi: 10.1007/s00277-016-2657-3. Epub 2016 Apr 27 [PubMed PMID: 27118542]
Feng X, McDonald JM. Disorders of bone remodeling. Annual review of pathology. 2011:6():121-45. doi: 10.1146/annurev-pathol-011110-130203. Epub [PubMed PMID: 20936937]
Level 3 (low-level) evidenceSmellie WS, Forth J, Ryder S, Galloway MJ, Wood AC, Watson ID. Best practice in primary care pathology: review 5. Journal of clinical pathology. 2006 Dec:59(12):1229-37 [PubMed PMID: 16644875]
Deeb A, Elfatih A. Could Alerting Physicians for Low Alkaline Phosphatase Levels Be Helpful in Early Diagnosis of Hypophosphatasia? Journal of clinical research in pediatric endocrinology. 2018 Mar 1:10(1):19-24. doi: 10.4274/jcrpe.4426. Epub 2017 Aug 2 [PubMed PMID: 28766503]
Morava E, Kárteszi J, Weisenbach J, Caliebe A, Mundlos S, Méhes K. Cleidocranial dysplasia with decreased bone density and biochemical findings of hypophosphatasia. European journal of pediatrics. 2002 Nov:161(11):619-22 [PubMed PMID: 12424590]
Level 3 (low-level) evidenceAgustina H, Asyifa I, Aziz A, Hernowo BS. The Role of Osteocalcin and Alkaline Phosphatase Immunohistochemistry in Osteosarcoma Diagnosis. Pathology research international. 2018:2018():6346409. doi: 10.1155/2018/6346409. Epub 2018 May 3 [PubMed PMID: 29854380]
Aparisi T, Arborgh B, Ericsson JL. Giant cell tumor of bone. Fine structural localization of alkaline phosphatase. Virchows Archiv. A, Pathological anatomy and histology. 1978 Jul 26:378(4):287-95 [PubMed PMID: 150116]
Level 3 (low-level) evidenceBreeland G, Sinkler MA, Menezes RG. Embryology, Bone Ossification. StatPearls. 2024 Jan:(): [PubMed PMID: 30969540]
Izumi Y. Alkaline phosphatase as a biochemical maturity index in female adolescence. Spine. 1993 Nov:18(15):2257-60 [PubMed PMID: 8278842]
Blumsohn A, Hannon RA, Wrate R, Barton J, al-Dehaimi AW, Colwell A, Eastell R. Biochemical markers of bone turnover in girls during puberty. Clinical endocrinology. 1994 May:40(5):663-70 [PubMed PMID: 7516828]
Level 2 (mid-level) evidenceBonjour JP, Chevalley T, Ferrari S, Rizzoli R. The importance and relevance of peak bone mass in the prevalence of osteoporosis. Salud publica de Mexico. 2009:51 Suppl 1():S5-17 [PubMed PMID: 19287894]
Lu J, Shin Y, Yen MS, Sun SS. Peak Bone Mass and Patterns of Change in Total Bone Mineral Density and Bone Mineral Contents From Childhood Into Young Adulthood. Journal of clinical densitometry : the official journal of the International Society for Clinical Densitometry. 2016 Apr-Jun:19(2):180-91. doi: 10.1016/j.jocd.2014.08.001. Epub 2014 Oct 18 [PubMed PMID: 25440183]
Chen JH, Liu C, You L, Simmons CA. Boning up on Wolff's Law: mechanical regulation of the cells that make and maintain bone. Journal of biomechanics. 2010 Jan 5:43(1):108-18. doi: 10.1016/j.jbiomech.2009.09.016. Epub 2009 Oct 8 [PubMed PMID: 19818443]
Teichtahl AJ, Wluka AE, Wijethilake P, Wang Y, Ghasem-Zadeh A, Cicuttini FM. Wolff's law in action: a mechanism for early knee osteoarthritis. Arthritis research & therapy. 2015 Sep 1:17(1):207. doi: 10.1186/s13075-015-0738-7. Epub 2015 Sep 1 [PubMed PMID: 26324398]
Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, Bikle DD. Vitamin D: Production, Metabolism and Mechanisms of Action. Endotext. 2000:(): [PubMed PMID: 25905172]
Chapuy MC, Durr F, Chapuy P. Age-related changes in parathyroid hormone and 25 hydroxycholecalciferol levels. Journal of gerontology. 1983 Jan:38(1):19-22 [PubMed PMID: 6600237]
Sözen T, Özışık L, Başaran NÇ. An overview and management of osteoporosis. European journal of rheumatology. 2017 Mar:4(1):46-56. doi: 10.5152/eurjrheum.2016.048. Epub 2016 Dec 30 [PubMed PMID: 28293453]
Level 3 (low-level) evidencePorter JL, Varacallo M. Osteoporosis. StatPearls. 2023 Jan:(): [PubMed PMID: 28722930]
Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, Rosen CJ. The Epidemiology and Pathogenesis of Osteoporosis. Endotext. 2000:(): [PubMed PMID: 25905357]
Akkawi I, Zmerly H. Osteoporosis: Current Concepts. Joints. 2018 Jun:6(2):122-127. doi: 10.1055/s-0038-1660790. Epub 2018 Jun 14 [PubMed PMID: 30051110]
Sheu A, Diamond T. Bone mineral density: testing for osteoporosis. Australian prescriber. 2016 Apr:39(2):35-9. doi: 10.18773/austprescr.2016.020. Epub 2016 Apr 1 [PubMed PMID: 27340320]
Mirza F, Canalis E. Management of endocrine disease: Secondary osteoporosis: pathophysiology and management. European journal of endocrinology. 2015 Sep:173(3):R131-51. doi: 10.1530/EJE-15-0118. Epub 2015 May 13 [PubMed PMID: 25971649]
Rosen CJ. Endocrine disorders and osteoporosis. Current opinion in rheumatology. 1997 Jul:9(4):355-61 [PubMed PMID: 9229183]
Level 3 (low-level) evidenceRobinson LJ, Yaroslavskiy BB, Griswold RD, Zadorozny EV, Guo L, Tourkova IL, Blair HC. Estrogen inhibits RANKL-stimulated osteoclastic differentiation of human monocytes through estrogen and RANKL-regulated interaction of estrogen receptor-alpha with BCAR1 and Traf6. Experimental cell research. 2009 Apr 15:315(7):1287-301. doi: 10.1016/j.yexcr.2009.01.014. Epub 2009 Jan 30 [PubMed PMID: 19331827]
Level 3 (low-level) evidenceXiong J, O'Brien CA. Osteocyte RANKL: new insights into the control of bone remodeling. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2012 Mar:27(3):499-505. doi: 10.1002/jbmr.1547. Epub [PubMed PMID: 22354849]
Level 3 (low-level) evidenceYasuda H. RANKL, a necessary chance for clinical application to osteoporosis and cancer-related bone diseases. World journal of orthopedics. 2013 Oct 18:4(4):207-17. doi: 10.5312/wjo.v4.i4.207. Epub 2013 Oct 18 [PubMed PMID: 24147256]
Bord S, Ireland DC, Beavan SR, Compston JE. The effects of estrogen on osteoprotegerin, RANKL, and estrogen receptor expression in human osteoblasts. Bone. 2003 Feb:32(2):136-41 [PubMed PMID: 12633785]
Kameda T, Mano H, Yuasa T, Mori Y, Miyazawa K, Shiokawa M, Nakamaru Y, Hiroi E, Hiura K, Kameda A, Yang NN, Hakeda Y, Kumegawa M. Estrogen inhibits bone resorption by directly inducing apoptosis of the bone-resorbing osteoclasts. The Journal of experimental medicine. 1997 Aug 18:186(4):489-95 [PubMed PMID: 9254647]
Level 3 (low-level) evidenceRiggs BL. The mechanisms of estrogen regulation of bone resorption. The Journal of clinical investigation. 2000 Nov:106(10):1203-4 [PubMed PMID: 11086020]
Level 3 (low-level) evidenceStreicher C, Heyny A, Andrukhova O, Haigl B, Slavic S, Schüler C, Kollmann K, Kantner I, Sexl V, Kleiter M, Hofbauer LC, Kostenuik PJ, Erben RG. Estrogen Regulates Bone Turnover by Targeting RANKL Expression in Bone Lining Cells. Scientific reports. 2017 Jul 25:7(1):6460. doi: 10.1038/s41598-017-06614-0. Epub 2017 Jul 25 [PubMed PMID: 28744019]
. Management of osteoporosis in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause (New York, N.Y.). 2010 Jan-Feb:17(1):25-54; quiz 55-6. doi: 10.1097/gme.0b013e3181c617e6. Epub [PubMed PMID: 20061894]
Cranney A, Papaioannou A, Zytaruk N, Hanley D, Adachi J, Goltzman D, Murray T, Hodsman A, Clinical Guidelines Committee of Osteoporosis Canada. Parathyroid hormone for the treatment of osteoporosis: a systematic review. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2006 Jul 4:175(1):52-9 [PubMed PMID: 16818910]
Level 1 (high-level) evidenceLou S, Lv H, Yin P, Li Z, Tang P, Wang Y. Combination therapy with parathyroid hormone analogs and antiresorptive agents for osteoporosis: a systematic review and meta-analysis of randomized controlled trials. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2019 Jan:30(1):59-70. doi: 10.1007/s00198-018-4790-4. Epub 2018 Dec 11 [PubMed PMID: 30539271]
Level 1 (high-level) evidenceDatta NS. Osteoporotic fracture and parathyroid hormone. World journal of orthopedics. 2011 Aug 18:2(8):67-74. doi: 10.5312/wjo.v2.i8.67. Epub [PubMed PMID: 22474638]
Martin TJ. Osteoblast-derived PTHrP is a physiological regulator of bone formation. The Journal of clinical investigation. 2005 Sep:115(9):2322-4 [PubMed PMID: 16138187]
Level 3 (low-level) evidenceByun JH, Jang S, Lee S, Park S, Yoon HK, Yoon BH, Ha YC. The Efficacy of Bisphosphonates for Prevention of Osteoporotic Fracture: An Update Meta-analysis. Journal of bone metabolism. 2017 Feb:24(1):37-49. doi: 10.11005/jbm.2017.24.1.37. Epub 2017 Feb 28 [PubMed PMID: 28326300]
Level 1 (high-level) evidenceReid IR. Bisphosphonates in the treatment of osteoporosis: a review of their contribution and controversies. Skeletal radiology. 2011 Sep:40(9):1191-6. doi: 10.1007/s00256-011-1164-9. Epub 2011 Aug 17 [PubMed PMID: 21847749]
Maruotti N, Corrado A, Cantatore FP. Osteoblast role in osteoarthritis pathogenesis. Journal of cellular physiology. 2017 Nov:232(11):2957-2963. doi: 10.1002/jcp.25969. Epub 2017 May 24 [PubMed PMID: 28425564]
Vakiti A, Anastasopoulou C, Mewawalla P. Malignancy-Related Hypercalcemia. StatPearls. 2023 Jan:(): [PubMed PMID: 29494030]
McCauley LK, Martin TJ. Twenty-five years of PTHrP progress: from cancer hormone to multifunctional cytokine. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2012 Jun:27(6):1231-9. doi: 10.1002/jbmr.1617. Epub 2012 May 1 [PubMed PMID: 22549910]
Level 3 (low-level) evidenceLuparello C. Parathyroid Hormone-Related Protein (PTHrP): A Key Regulator of Life/Death Decisions by Tumor Cells with Potential Clinical Applications. Cancers. 2011 Jan 20:3(1):396-407. doi: 10.3390/cancers3010396. Epub 2011 Jan 20 [PubMed PMID: 24212621]
Soki FN, Park SI, McCauley LK. The multifaceted actions of PTHrP in skeletal metastasis. Future oncology (London, England). 2012 Jul:8(7):803-17. doi: 10.2217/fon.12.76. Epub [PubMed PMID: 22830401]
Suva LJ, Washam C, Nicholas RW, Griffin RJ. Bone metastasis: mechanisms and therapeutic opportunities. Nature reviews. Endocrinology. 2011 Apr:7(4):208-18. doi: 10.1038/nrendo.2010.227. Epub 2011 Jan 4 [PubMed PMID: 21200394]
Shupp AB, Kolb AD, Mukhopadhyay D, Bussard KM. Cancer Metastases to Bone: Concepts, Mechanisms, and Interactions with Bone Osteoblasts. Cancers. 2018 Jun 4:10(6):. doi: 10.3390/cancers10060182. Epub 2018 Jun 4 [PubMed PMID: 29867053]
Mannstadt M, Jüppner H, Gardella TJ. Receptors for PTH and PTHrP: their biological importance and functional properties. The American journal of physiology. 1999 Nov:277(5):F665-75. doi: 10.1152/ajprenal.1999.277.5.F665. Epub [PubMed PMID: 10564229]
Level 3 (low-level) evidenceLi J, Karaplis AC, Huang DC, Siegel PM, Camirand A, Yang XF, Muller WJ, Kremer R. PTHrP drives breast tumor initiation, progression, and metastasis in mice and is a potential therapy target. The Journal of clinical investigation. 2011 Dec:121(12):4655-69. doi: 10.1172/JCI46134. Epub 2011 Nov 7 [PubMed PMID: 22056386]
Level 3 (low-level) evidenceYang Y, Wang B. PTH1R-CaSR Cross Talk: New Treatment Options for Breast Cancer Osteolytic Bone Metastases. International journal of endocrinology. 2018:2018():7120979. doi: 10.1155/2018/7120979. Epub 2018 Jul 29 [PubMed PMID: 30151009]
Carter PH, Schipani E. The roles of parathyroid hormone and calcitonin in bone remodeling: prospects for novel therapeutics. Endocrine, metabolic & immune disorders drug targets. 2006 Mar:6(1):59-76 [PubMed PMID: 16611165]
Level 3 (low-level) evidenceMigliaccio S, Brama M, Spera G. The differential effects of bisphosphonates, SERMS (selective estrogen receptor modulators), and parathyroid hormone on bone remodeling in osteoporosis. Clinical interventions in aging. 2007:2(1):55-64 [PubMed PMID: 18044075]
Weinstein RS, Chen JR, Powers CC, Stewart SA, Landes RD, Bellido T, Jilka RL, Parfitt AM, Manolagas SC. Promotion of osteoclast survival and antagonism of bisphosphonate-induced osteoclast apoptosis by glucocorticoids. The Journal of clinical investigation. 2002 Apr:109(8):1041-8 [PubMed PMID: 11956241]
Level 3 (low-level) evidenceBecker DE. Basic and clinical pharmacology of glucocorticosteroids. Anesthesia progress. 2013 Spring:60(1):25-31; quiz 32. doi: 10.2344/0003-3006-60.1.25. Epub [PubMed PMID: 23506281]