Introduction
In the early nineteenth century, Charles Dickens reported in his book “The Posthumous Papers of the Pickwick Club” on the obesity hypoventilation syndrome (OHS), which is defined as alveolar hypoventilation in an obese individual during wakefulness that cannot be explained to other conditions that lead to hypercapnia such as chronic obstructive lung disease (COPD).[1] OHS is linked to significant cardiorespiratory morbidity and mortality with limited options for therapy. The exact pathogenesis of HS is unknown; however, reduced hypercapnic ventilatory response plays an important role. The etiology, epidemiology, pathophysiology, clinical manifestations, and evaluation are discussed here. Other types of sleep-disordered breathing are discussed in the following sections.[2][3]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
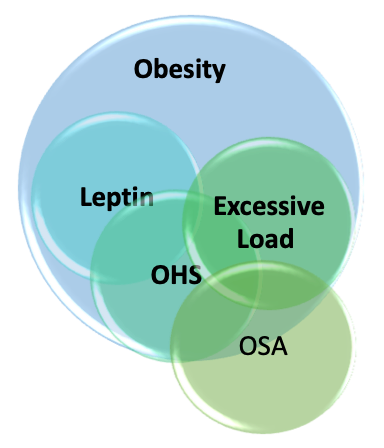
The responsible causes for OHS are multifactorial, with obesity and obstructive sleep apnea as the leading causes; other contributing factors involved in the etiology of OHS include ventilatory control defects leading to decreased responsiveness in the hypoxic and hypercapnic ventilatory drive, as outlined in the pathophysiology section.[4]
Epidemiology
The prevalence of OHS is reported to have a highly variable prevalence in published studies. There is a global epidemic of obesity affecting all ages, including children, adolescents, and adults; hence the prevalence of OHS is also increasing in these age groups. Currently, 35% of the United States population suffers from obesity, and the prevalence of morbid obesity (body mass index (BMI) > 40 kg/m^2).[5][6][7] The prevalence of morbid obesity increased five-fold, and estimates are that 8% of the adult U.S. population has morbid obesity.[8] Likewise, the prevalence of extreme obesity (BMI>50 kg/m^2 has increased 10-fold and is rising.[9] The prevalence in the population having obstructive sleep apnea (OSA) is estimated to be between 20 to 30%.[10]
The incidence rate of OHS is seen at higher rates in men than in women, older individuals, and African Americans than in the White race population.[11] In addition, OHS is known to occur at a lower BMI range in the Asian community.[12]
The prevalence of OHS has been reported to be higher in men; however, among patients referred to the sleep disorders clinic, OHS was more prevalent in women than men.[13] The delay in identifying OHS in women was linked to a worse and more advanced consequence of the disease.[14]
Pathophysiology
Obesity hypoventilation syndrome (OHS) occurs due to complex interactions between multiple pathological processes, including diminished respiratory drive, structural and functional respiratory impairment, and sleep-related breathing alterations. Chronic steady-state hypercapnia occurs due to the failure of compensatory ventilatory mechanisms.[5][15][16][17] Obesity-related increased CO2 production (VCO2) is an independent variable of arterial PaCO2 level, based on the alveolar ventilation (VA) equations: PaCO2=K VCO2/VA, (K is a constant ) and VA is minute ventilation (VE) minus dead space ventilation (VD). In contrast, decreased alveolar ventilation due to any cause (obesity, atelectasis, or mechanical loading) leads to increased PaCO2.
Obesity
Respiratory system mechanics are affected significantly by obesity and fat distribution.[18] The daily increase in PaCO2 in obese individuals was a strong respiratory determinant of hypercapnia in OHS. [19] Furthermore, hypercapnic patients had significantly greater CO2 production as a cause for hypercapnia. However, when adjusting for body surface area, the CO2 production was similar between those with OSA and hypercapnia. They do not have hypercapnia (52 vs. 40 mmHg), matched for apnea-hypopnea index.
Excessive Load on Respiratory System
Respiratory Muscles
The maximal inspiratory and expiratory pressures are reduced in patients with OHS while normal in morbidly obese patients with eucapnic or mild OHS.[20][21] In addition, patients with OHS who have hypercapnia could generate trans-diaphragmatic pressure similar to patients with obesity during hypercapnia-induced hyperventilation, suggesting that reduced ventilatory drive, not mechanical limitations, contribute to the pathogenesis of OHS.[22]
Respiratory Mechanics
Due to reduced pulmonary distensibility, obese patients suffer reduced ventilation in the lower pulmonary lobes. The alveoli close before the expiration, thus producing a characteristic breathing pattern of low tidal volume and an increased respiratory rate, causing an increase in the dead ventilation space. Decreased ventilation of the lower lobes causes alterations in the ventilation-perfusion (V/Q), thus triggering hypoxemia. Total lung capacity (TLC), expiratory reserve volume (ERV), and residual functional capacity (RFC) are reduced in patients with OHS as opposed to eucapnic obese patients.
Respiratory Drive
Patients with OHS have a blunted respiratory drive in response to a hypercapnic challenge. Multiple possible pathogenic mechanisms have been proposed to explain the blunted respiratory response, including possible leptin resistance, genetic predisposition, and sleep-disordered breathing.
Intrinsically diminished chemosensitivity to CO2 retention has been reported in OHS patients. It is possible that this diminished hypoxic and hypercapnic chemosensitivity could be the underlying reason for hypoventilation in patients with idiopathic obesity hypoventilation syndrome.
Leptin
Leptin is a 16-kDa protein encoded by the ob gene discovered in 1994. [23] Leptin is produced in the adipose tissue and regulates appetite, and stimulates ventilation.[24] The excess fatty tissue associated with obesity leads to increasing levels of leptin which can prevent ventilatory depression.[25] For leptin to increase ventilation, the level rises to compensate for increased respiratory demand.[26] Therefore, patients with OHS and OSA have higher leptin levels and higher leptin resistance than matched control subjects without OSA. [27] Likewise, compared with eupneic patients with OSA, patients with OHS have higher serum levels of leptin.[28]
OSA
An estimated 90% of patients with OHS have obstructive sleep apnea (OSA) (defined by an apnea-hypopnea index-AHI ≥ five events/hour), and the majority (approximately 70%) are severe (AHI ≥ 30 events/hour).[29] However, 10% of OHS patients without OSA are due to sleep-related hypoventilation.
The PaCO2 increase is secondary to the cessation of ventilation during apneic events and the continued metabolic production of CO2. Eucapnic patients can normalize the PaCO2 levels via compensatory augmentation of alveolar ventilation, which increases CO2 clearance. However, in OHS patients, the compensatory mechanism is disrupted, causing the retention of CO2.[30] In response to transitory hypercapnia, the renal system decreases bicarbonate clearance to compensate for the hypercapnic pH drop. This built-up in bicarbonate eventually blunts the ventilatory response to carbon dioxide, thus causing the development of nocturnal hypoventilation.
Sleep Hypoventilation
Almost 5% to 10 % of patients with OHS have sleep hypoventilation and a PaCO2 elevation during sleep of 10 mm Hg or higher. These patients are clinically indistinguishable from those with concomitant OSA. Sustained hypoxia significantly delays the warning signals of decreased ventilation and could potentially contribute to hypoventilation.
Given its extensive magnitude, addressing the associated metabolic, cardiovascular, and respiratory complications is paramount.[31][32][33]
History and Physical
While some patients with OHS present with acute chronic exacerbation of respiratory failure with acute respiratory acidosis, others remain clinically stable at diagnosis. The majority of patients have classic symptoms of OSA, including loud snoring, nocturnal choking episodes with witnessed apneas, excessive daytime sleepiness, and morning headaches, as well as hypoxemia during wake and more severe hypoxemia during sleep. Patients often exhibit dyspnea and may have signs of cor pulmonale. Classical physical examination findings include an enlarged neck circumference, a crowded oropharynx, a prominent pulmonic component of the second heart sound on cardiac auscultation, and lower extremity edema.
Evaluation
Clinical suspicion should be high in patients with BMI > 30 kg/m^2 with unexplained dyspnea on exertion and hypersomnolence.[33] Hence, screening for OHS in severe obesity and OSA patients is recommended. Additionally, patients with evidence of wakefulness hypoxia on room air or reduced pulse oximetry (SpO2) <94% and/or increased serum bicarbonate level >27 mEq/L should be suspected to have OHS.[10] The elevated serum bicarbonate level, typically seen as a result of metabolic compensation of respiratory acidosis, points toward the chronic nature of hypercapnia. A serum bicarbonate level can sometimes serve as a sensitive test to screen for chronic hypercapnia. In patients with serum bicarbonate>27 mEq/L, OHS was present in 50%. On the other hand, when serum bicarbonate level <27 mmol/L, it excludes the diagnosis of OHS when the pre-test probability is not very high (<20%).[34]
Ultimately, an arterial blood gas should show hypoventilation in complete wakefulness. The percent of total sleep time with SpO2 spent below 90% can be a useful polysomnographic variable for the evaluation of OHS patients. Moreover, patients with very severe OSA (AHI > 100 events/h) or severe hypoxia (Nadir SpO2 <60%) during sleep had increased the prevalence of OHS by more than 75%.[10]
The American Academy of Sleep Medicine introduced criteria for the diagnosis of OHS, which include:
- Presence of hypoventilation during wakefulness (PaCO2 more significant than 45 mmHg) as measured by arterial PCO2, end-tidal PCO2, or transcutaneous PCO2.[35]
- Presence of obesity (body mass index or BMI greater than 30 kg/m^2; more significant than the 95th percentile for age and sex for children).[35]
- Hypoventilation is not primarily due to lung parenchymal or airway disease, pulmonary vascular pathology, chest wall disorder (other than mass loading from obesity), medication use, neurologic disorder, muscle weakness, or a known congenital or idiopathic central alveolar hypoventilation syndrome.[35]
The recommended diagnostic approach is to demonstrate daytime hypoventilation. An arterial blood gas analysis is the most definitive diagnostic test for alveolar hypoventilation. Unfortunately, an ABG is not readily done in an outpatient setting. However, in most patients with OHS, the most common initial presentation is in a hospital setting after presenting with an acute exacerbation, where an ABG and a basic metabolic panel can be done. Polysomnography is not required for diagnosis but helps distinguish patients with coexistent OSA and those with actual sleep hypoventilation. Sleep hypoventilation is a ten mmHg increase in PaCO2 above wakefulness that is not secondary to obstructive apneas or hypopnea.
OHS is a diagnosis of exclusion that requires to be distinguished from disorders that are also associated with hypoventilation. Once hypercapnia is confirmed by ABG, pulmonary function testing should be performed to exclude other hypercapnia causes. In these patients with OHS, pulmonary function tests are usually within normal or consistent with the evidence of restrictive ventilatory defect without significant evidence of airflow obstruction.
Treatment / Management
OHS is associated with a significantly high rate of morbidity and mortality. Although treatment modalities target different aspects of the underlying pathophysiology, the goal is to normalize arterial CO2 hypoxia and improve symptoms. Several therapeutic options have been tried, including positive airway pressure therapy, weight reduction surgery, and pharmacotherapy.[36][37] (B2)
Positive Airway Pressure Therapy
Positive airway pressure (PAP) therapy is typically the first-line treatment for OHS.[38] PAP therapy significantly reduces the nocturnal build-up of PaCO2 and improves sleepiness during the daytime. Treatment options include continuous positive airway pressure (CPAP), bi-level PAP, and other noninvasive ventilation (NIV) modalities. The current recommendation is to use CPAP rather than NIV if concomitant severe obstructive sleep apnea is present in stable ambulatory patients.[34] NIV can be beneficial in patients with hypercapnia without significant apnea or hypopnea or if the patient did not tolerate PAP or did not respond to CPAP as initial therapy.[34] However, NIV should be used in hospitalized patients with acute, chronic hypercapnia respiratory failure suspected of having OHS until they undergo outpatient diagnostic procedures and PAP titration in the sleep laboratory.[38][39] (A1)
A recent study compared the three standard treatments for OHS, including NIV, CPAP, and lifestyle modification. This study showed that both NIV and CPAP significantly improved polysomnographic parameters, although NIV was superior in improving respiratory parameters compared to other treatment modalities. In a total of 351 patients compared to baseline, at two months, the three treatments showed a reduction in PaCO2 of 5.5, 3.7, and 3.2 with NIV, CPAP, and lifestyle modification, respectively.
Weight Reduction
Weight loss can improve both OHS and OSA and cardiovascular comorbidities.[40] Numerous studies have shown improvement in OHS symptoms with weight reduction.[41] Weight loss significantly reduces CO2 production and improves sleep apnea severity and alveolar ventilation. It also improves pulmonary artery hypertension and left ventricular dysfunction, significantly reducing cardiovascular compromise in OHS patients. Given that therapeutic goals of weight loss require interventions that produce 25% to 30% of total body weight, surgical options are recommended if not contraindicated (e,g bariatric surgeries).[42][34](A1)
Tracheostomy
Tracheostomy relieves airway obstruction during sleep, thus improving alveolar ventilation and waking PaCO2.[27] However, some patients may not return to a eucapnic state post tracheostomy, as it does not affect CO2 production and impaired muscle strength.
Further details on the management of OHS and Pickwickian Syndrome can be found in another section.[30]
Differential Diagnosis
Limitation to Ventilation
- Chest wall diseases
- Neuromuscular diseases
- Obstructive lung disease
Central Control Defects
- Congenital central hypoventilation (Ondine’s curse)
- PHOX2B mutation on chromosome 4p12
- Brainstem lesions
- Carotid body disease
- Metabolic alkalosis
Combined Defects
- Chronic obstructive pulmonary disease (COPD)
- Hypothyroidism
- Sleep apnea
Prognosis
Obesity hypoventilation is associated with reduced quality of life and prolonged admission rates and time in the intensive care unit.[43] In patients with other medical conditions, such as diabetes and asthma, the mortality rates are significantly high, with 23% over 18 months and 46% over 50 months. Early use of CPAP can reduce the associated mortality by 10%. The baseline PaCO2 is an independent predictor for persistent hypoventilation despite PAP treatment.[43]
Likewise, older patients with restrictive ventilatory defects (on pulmonary function test) or recent acute on chronic hypercapnia respiratory failure may not respond adequately to CPAP.[44] The prognosis is poor for patients with OHS who do not lose weight, with a shortened life expectancy.[45][46]
Complications
Obesity is an independent risk factor for pulmonary hypertension (PH) in OHS with and without concomitant OSA.[47] A recent post hoc assessment of the Pickwick trial (N=246) reported that 50% of patients with OHS had evidence of PH on echocardiography (defined as systolic pulmonary artery pressure of 40 mmHg or more) risk factors associated with pulmonary hypertension.[47] This study found that both low PaO2 levels during wakefulness and obesity are independent predictors of PH in severe OSA phenotypes. In contrast, obesity and early/late diastolic relationship were predictors of PH in the non-severe OSA group.
Postoperative and Rehabilitation Care
Patients with OHS have higher rates of comorbidities than those with OSA alone, which can lead to a higher risk for postoperative complications.[48] Therefore, patients with obesity and/or OSA, hypoventilation, or unexplained hypoxemia require careful monitoring and assessment postoperatively. This includes caution in selecting sedation and anesthesia, the position of patients, and appropriate use of oxygen and non-invasive ventilation.
Deterrence and Patient Education
As discussed in this article, patent awareness of obesity hypoventilation syndrome is essential, and the importance of early evaluation and adherence to treatment is critical. Patients will need to be aware of the available option of therapy from weight loss targets, options beyond diet and exercise alone (such as surgical and bariatric treatments), and how to use their mechanical devices (such as positive airway pressure and non-invasive ventilation) correctly.
Pearls and Other Issues
- Obesity is associated with multiple medical complications. Obesity hypoventilation syndrome is one of the significant respiratory consequences related to obesity.
- The presence of hypoventilation during wakefulness with PaCO2 more significant than 45 mm Hg in the presence of obesity (BMI greater than 30 kg/m^2) confirms the diagnosis, given that hypoventilation is not due to lung parenchymal or airway disease, pulmonary vascular pathology, or chest wall disorder.
- Therefore, serum bicarbonate levels can be a sensitive test to screen for chronic hypercapnia.
- The percent of total sleep time with SpO2 spent below 90% can be a useful polysomnographic variable for evaluating OHS patients.
- Positive airway pressure therapy is the first-line treatment for OHS and OSA, which significantly reduces the nocturnal build-up of PaCO2 and improves sleepiness during the daytime.
Enhancing Healthcare Team Outcomes
Obesity is best managed with an interprofessional team, including clinicians (including mid-level practitioners and specialists), dietitians, nurses, therapists, and pharmacists. Each team member should be able to contribute from their specialty to manage the case. For example, nurses will assist in patient evaluation, coordinate activities between various clinicians, and offer patient counseling. The dietician will make recommendations for a nutrition plan to enable the patient to lose weight, while the pharmacist can assist in selecting a weight-loss medication. Obesity has significant morbidity and mortality if left untreated. Psychological professionals can also help the patient with the behavioral changes needed. The key is to educate the patient on the harms of obesity. Patients must change their lifestyle, become physically active, maintain a healthy weight, and exercise regularly. All current therapies for obesity hypoventilation syndrome are palliative until the patient loses weight. Under a coordinated interprofessional team effort with open communication, this becomes increasingly possible. [Level 5]
Media
(Click Image to Enlarge)
References
Sankri-Tarbichi AG. Obstructive sleep apnea-hypopnea syndrome: Etiology and diagnosis. Avicenna journal of medicine. 2012 Jan:2(1):3-8. doi: 10.4103/2231-0770.94803. Epub [PubMed PMID: 23210013]
Maggard MD, Sankari A, Cascella M. Upper Airway Resistance Syndrome. StatPearls. 2024 Jan:(): [PubMed PMID: 33232072]
Rana AM, Sankari A. Central Sleep Apnea. StatPearls. 2023 Jan:(): [PubMed PMID: 35201727]
Zwillich CW, Sutton FD, Pierson DJ, Greagh EM, Weil JV. Decreased hypoxic ventilatory drive in the obesity-hypoventilation syndrome. The American journal of medicine. 1975 Sep:59(3):343-8 [PubMed PMID: 1163544]
Athayde RAB, Oliveira Filho JRB, Lorenzi Filho G, Genta PR. Obesity hypoventilation syndrome: a current review. Jornal brasileiro de pneumologia : publicacao oficial da Sociedade Brasileira de Pneumologia e Tisilogia. 2018 Nov-Dec:44(6):510-518. doi: 10.1590/S1806-37562017000000332. Epub [PubMed PMID: 30726328]
Nicolini A, Ferrando M, Solidoro P, Di Marco F, Facchini F, Braido F. Non-invasive ventilation in acute respiratory failure of patients with obesity hypoventilation syndrome. Minerva medica. 2018 Dec:109(6 Suppl 1):1-5. doi: 10.23736/S0026-4806.18.05921-9. Epub [PubMed PMID: 30642143]
Markussen H, Lehmann S, Nilsen RM, Natvig GK. Health-related quality of life as predictor for mortality in patients treated with long-term mechanical ventilation. BMC pulmonary medicine. 2019 Jan 11:19(1):13. doi: 10.1186/s12890-018-0768-4. Epub 2019 Jan 11 [PubMed PMID: 30635052]
Level 2 (mid-level) evidenceHales CM, Fryar CD, Carroll MD, Freedman DS, Aoki Y, Ogden CL. Differences in Obesity Prevalence by Demographic Characteristics and Urbanization Level Among Adults in the United States, 2013-2016. JAMA. 2018 Jun 19:319(23):2419-2429. doi: 10.1001/jama.2018.7270. Epub [PubMed PMID: 29922829]
Sturm R. Increases in morbid obesity in the USA: 2000-2005. Public health. 2007 Jul:121(7):492-6 [PubMed PMID: 17399752]
Mokhlesi B, Tulaimat A, Faibussowitsch I, Wang Y, Evans AT. Obesity hypoventilation syndrome: prevalence and predictors in patients with obstructive sleep apnea. Sleep & breathing = Schlaf & Atmung. 2007 Jun:11(2):117-24 [PubMed PMID: 17187265]
Level 2 (mid-level) evidenceKessler R, Chaouat A, Schinkewitch P, Faller M, Casel S, Krieger J, Weitzenblum E. The obesity-hypoventilation syndrome revisited: a prospective study of 34 consecutive cases. Chest. 2001 Aug:120(2):369-76 [PubMed PMID: 11502631]
Level 3 (low-level) evidenceIftikhar IH, Roland J. Obesity Hypoventilation Syndrome. Clinics in chest medicine. 2018 Jun:39(2):427-436. doi: 10.1016/j.ccm.2018.01.006. Epub [PubMed PMID: 29779600]
BaHammam AS, Pandi-Perumal SR, Piper A, Bahammam SA, Almeneessier AS, Olaish AH, Javaheri S. Gender differences in patients with obesity hypoventilation syndrome. Journal of sleep research. 2016 Aug:25(4):445-53. doi: 10.1111/jsr.12400. Epub 2016 Mar 18 [PubMed PMID: 26990045]
Palm A, Midgren B, Janson C, Lindberg E. Gender differences in patients starting long-term home mechanical ventilation due to obesity hypoventilation syndrome. Respiratory medicine. 2016 Jan:110():73-8. doi: 10.1016/j.rmed.2015.11.010. Epub 2015 Nov 26 [PubMed PMID: 26680503]
Ballard HA, Leavitt OS, Chin AC, Kabre R, Weese-Mayer DE, Hajduk J, Jagannathan N. Perioperative anesthetic management of children with congenital central hypoventilation syndrome and rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation undergoing thoracoscopic phrenic nerve-diaphragm pacemaker implantation. Paediatric anaesthesia. 2018 Nov:28(11):963-973. doi: 10.1111/pan.13475. Epub 2018 Sep 24 [PubMed PMID: 30251310]
Lemyze M, Guiot A, Mallat J, Thevenin D. The obesity supine death syndrome (OSDS). Obesity reviews : an official journal of the International Association for the Study of Obesity. 2018 Apr:19(4):550-556. doi: 10.1111/obr.12655. Epub 2017 Dec 13 [PubMed PMID: 29239066]
Piper AJ, BaHammam AS, Javaheri S. Obesity Hypoventilation Syndrome: Choosing the Appropriate Treatment of a Heterogeneous Disorder. Sleep medicine clinics. 2017 Dec:12(4):587-596. doi: 10.1016/j.jsmc.2017.07.008. Epub 2017 Sep 6 [PubMed PMID: 29108613]
Dixon AE, Peters U. The effect of obesity on lung function. Expert review of respiratory medicine. 2018 Sep:12(9):755-767. doi: 10.1080/17476348.2018.1506331. Epub 2018 Aug 14 [PubMed PMID: 30056777]
Javaheri S, Simbartl LA. Respiratory determinants of diurnal hypercapnia in obesity hypoventilation syndrome. What does weight have to do with it? Annals of the American Thoracic Society. 2014 Jul:11(6):945-50. doi: 10.1513/AnnalsATS.201403-099OC. Epub [PubMed PMID: 24828690]
Han F, Chen E, Wei H, He Q, Ding D, Strohl KP. Treatment effects on carbon dioxide retention in patients with obstructive sleep apnea-hypopnea syndrome. Chest. 2001 Jun:119(6):1814-9 [PubMed PMID: 11399709]
Kelly TM, Jensen RL, Elliott CG, Crapo RO. Maximum respiratory pressures in morbidly obese subjects. Respiration; international review of thoracic diseases. 1988:54(2):73-7 [PubMed PMID: 3231898]
Sampson MG, Grassino K. Neuromechanical properties in obese patients during carbon dioxide rebreathing. The American journal of medicine. 1983 Jul:75(1):81-90 [PubMed PMID: 6407317]
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994 Dec 1:372(6505):425-32 [PubMed PMID: 7984236]
Level 3 (low-level) evidenceAmorim MR, Aung O, Mokhlesi B, Polotsky VY. Leptin-mediated neural targets in obesity hypoventilation syndrome. Sleep. 2022 Sep 8:45(9):. doi: 10.1093/sleep/zsac153. Epub [PubMed PMID: 35778900]
O'donnell CP, Schaub CD, Haines AS, Berkowitz DE, Tankersley CG, Schwartz AR, Smith PL. Leptin prevents respiratory depression in obesity. American journal of respiratory and critical care medicine. 1999 May:159(5 Pt 1):1477-84 [PubMed PMID: 10228114]
Level 3 (low-level) evidenceMakinodan K, Yoshikawa M, Fukuoka A, Tamaki S, Koyama N, Yamauchi M, Tomoda K, Hamada K, Kimura H. Effect of serum leptin levels on hypercapnic ventilatory response in obstructive sleep apnea. Respiration; international review of thoracic diseases. 2008:75(3):257-64 [PubMed PMID: 18073454]
Piper AJ, Grunstein RR. Obesity hypoventilation syndrome: mechanisms and management. American journal of respiratory and critical care medicine. 2011 Feb 1:183(3):292-8. doi: 10.1164/rccm.201008-1280CI. Epub 2010 Oct 29 [PubMed PMID: 21037018]
Phipps PR, Starritt E, Caterson I, Grunstein RR. Association of serum leptin with hypoventilation in human obesity. Thorax. 2002 Jan:57(1):75-6 [PubMed PMID: 11809994]
Masa JF, Corral J, Alonso ML, Ordax E, Troncoso MF, Gonzalez M, Lopez-Martínez S, Marin JM, Marti S, Díaz-Cambriles T, Chiner E, Aizpuru F, Egea C, Spanish Sleep Network. Efficacy of Different Treatment Alternatives for Obesity Hypoventilation Syndrome. Pickwick Study. American journal of respiratory and critical care medicine. 2015 Jul 1:192(1):86-95. doi: 10.1164/rccm.201410-1900OC. Epub [PubMed PMID: 25915102]
Ghimire P, Sankari A, Kaul P. Pickwickian Syndrome. StatPearls. 2023 Jan:(): [PubMed PMID: 31194373]
Murphy PB, Suh ES, Hart N. Non-invasive ventilation for obese patients with chronic respiratory failure: Are two pressures always better than one? Respirology (Carlton, Vic.). 2019 Oct:24(10):952-961. doi: 10.1111/resp.13588. Epub 2019 May 23 [PubMed PMID: 31121638]
Orr JE, Coleman J, Criner GJ, Sundar KM, Tsai SC, Benjafield AV, Crocker ME, Willes L, Malhotra A, Owens RL, Wolfe LF. Automatic EPAP intelligent volume-assured pressure support is effective in patients with chronic respiratory failure: A randomized trial. Respirology (Carlton, Vic.). 2019 Dec:24(12):1204-1211. doi: 10.1111/resp.13546. Epub 2019 Apr 22 [PubMed PMID: 31012225]
Level 1 (high-level) evidenceMasa JF, Pépin JL, Borel JC, Mokhlesi B, Murphy PB, Sánchez-Quiroga MÁ. Obesity hypoventilation syndrome. European respiratory review : an official journal of the European Respiratory Society. 2019 Mar 31:28(151):. doi: 10.1183/16000617.0097-2018. Epub 2019 Mar 14 [PubMed PMID: 30872398]
Mokhlesi B, Masa JF, Brozek JL, Gurubhagavatula I, Murphy PB, Piper AJ, Tulaimat A, Afshar M, Balachandran JS, Dweik RA, Grunstein RR, Hart N, Kaw R, Lorenzi-Filho G, Pamidi S, Patel BK, Patil SP, Pépin JL, Soghier I, Tamae Kakazu M, Teodorescu M. Evaluation and Management of Obesity Hypoventilation Syndrome. An Official American Thoracic Society Clinical Practice Guideline. American journal of respiratory and critical care medicine. 2019 Aug 1:200(3):e6-e24. doi: 10.1164/rccm.201905-1071ST. Epub [PubMed PMID: 31368798]
Level 1 (high-level) evidenceLemyze M, De Palleja G, Guiot A, Bury Q, Jonard M, Granier M, Thevenin D, Mallat J. Outcome of Frail Do-Not-Intubate Subjects With End-Stage Chronic Respiratory Failure and Their Opinion of Noninvasive Ventilation to Reverse Hypercapnic Coma. Respiratory care. 2019 Sep:64(9):1023-1030. doi: 10.4187/respcare.06346. Epub 2019 Mar 19 [PubMed PMID: 30890633]
Level 3 (low-level) evidenceMacIntyre EJ, Asadi L, Mckim DA, Bagshaw SM. Clinical Outcomes Associated with Home Mechanical Ventilation: A Systematic Review. Canadian respiratory journal. 2016:2016():6547180. doi: 10.1155/2016/6547180. Epub 2016 Apr 28 [PubMed PMID: 27445559]
Level 2 (mid-level) evidenceCarron M, Zarantonello F, Ieppariello G, Ori C. Obesity and perioperative noninvasive ventilation in bariatric surgery. Minerva chirurgica. 2017 Jun:72(3):248-264. doi: 10.23736/S0026-4733.17.07310-2. Epub [PubMed PMID: 28482650]
Gong Y, Sankari A. Noninvasive Ventilation. StatPearls. 2024 Jan:(): [PubMed PMID: 35201716]
Daoud A, Haider S, Sankari A. Noninvasive Ventilation and Spinal Cord Injury. Sleep medicine clinics. 2020 Dec:15(4):461-470. doi: 10.1016/j.jsmc.2020.08.006. Epub 2020 Oct 6 [PubMed PMID: 33131657]
Look AHEAD Research Group, Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM, Espeland MA, Evans M, Foreyt JP, Ghazarian S, Gregg EW, Harrison B, Hazuda HP, Hill JO, Horton ES, Hubbard VS, Jakicic JM, Jeffery RW, Johnson KC, Kahn SE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montez MG, Murillo A, Nathan DM, Patricio J, Peters A, Pi-Sunyer X, Pownall H, Reboussin D, Regensteiner JG, Rickman AD, Ryan DH, Safford M, Wadden TA, Wagenknecht LE, West DS, Williamson DF, Yanovski SZ. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. The New England journal of medicine. 2013 Jul 11:369(2):145-54. doi: 10.1056/NEJMoa1212914. Epub 2013 Jun 24 [PubMed PMID: 23796131]
Level 1 (high-level) evidenceGudzune KA, Doshi RS, Mehta AK, Chaudhry ZW, Jacobs DK, Vakil RM, Lee CJ, Bleich SN, Clark JM. Efficacy of commercial weight-loss programs: an updated systematic review. Annals of internal medicine. 2015 Apr 7:162(7):501-12. doi: 10.7326/M14-2238. Epub [PubMed PMID: 25844997]
Level 1 (high-level) evidenceKakazu MT, Soghier I, Afshar M, Brozek JL, Wilson KC, Masa JF, Mokhlesi B. Weight Loss Interventions as Treatment of Obesity Hypoventilation Syndrome. A Systematic Review. Annals of the American Thoracic Society. 2020 Apr:17(4):492-502. doi: 10.1513/AnnalsATS.201907-554OC. Epub [PubMed PMID: 31978317]
Level 1 (high-level) evidenceHoward ME, Piper AJ, Stevens B, Holland AE, Yee BJ, Dabscheck E, Mortimer D, Burge AT, Flunt D, Buchan C, Rautela L, Sheers N, Hillman D, Berlowitz DJ. A randomised controlled trial of CPAP versus non-invasive ventilation for initial treatment of obesity hypoventilation syndrome. Thorax. 2017 May:72(5):437-444. doi: 10.1136/thoraxjnl-2016-208559. Epub 2016 Nov 15 [PubMed PMID: 27852952]
Level 1 (high-level) evidenceSoghier I, Brożek JL, Afshar M, Tamae Kakazu M, Wilson KC, Masa JF, Mokhlesi B. Noninvasive Ventilation versus CPAP as Initial Treatment of Obesity Hypoventilation Syndrome. Annals of the American Thoracic Society. 2019 Oct:16(10):1295-1303. doi: 10.1513/AnnalsATS.201905-380OC. Epub [PubMed PMID: 31365842]
Oga T, Windisch W, Handa T, Hirai T, Chin K. Health-related quality of life measurement in patients with chronic respiratory failure. Respiratory investigation. 2018 May:56(3):214-221. doi: 10.1016/j.resinv.2018.01.006. Epub 2018 Mar 9 [PubMed PMID: 29773292]
Level 2 (mid-level) evidenceKauppert CA, Dvorak I, Kollert F, Heinemann F, Jörres RA, Pfeifer M, Budweiser S. Pulmonary hypertension in obesity-hypoventilation syndrome. Respiratory medicine. 2013 Dec:107(12):2061-70. doi: 10.1016/j.rmed.2013.09.017. Epub 2013 Sep 28 [PubMed PMID: 24120252]
Level 2 (mid-level) evidenceMasa JF, Benítez ID, Javaheri S, Mogollon MV, Sánchez-Quiroga MÁ, de Terreros FJG, Corral J, Gallego R, Romero A, Caballero-Eraso C, Ordax-Carbajo E, Troncoso MF, González M, López-Martín S, Marin JM, Martí S, Díaz-Cambriles T, Chiner E, Egea C, Barca J, Barbé F, Mokhlesi B, Spanish Sleep Network. Risk factors associated with pulmonary hypertension in obesity hypoventilation syndrome. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2022 Apr 1:18(4):983-992. doi: 10.5664/jcsm.9760. Epub [PubMed PMID: 34755598]
Kaw R, Wong J, Mokhlesi B. Obesity and Obesity Hypoventilation, Sleep Hypoventilation, and Postoperative Respiratory Failure. Anesthesia and analgesia. 2021 May 1:132(5):1265-1273. doi: 10.1213/ANE.0000000000005352. Epub [PubMed PMID: 33857968]