Indications
Reports of acute and chronic kidney diseases are increasing in the U.S. and various parts of the world. When looking for a critical metric to understand when not to treat using a nephrotoxic medication, the standard is set at an eGFR less than 60 mL/min per 1.73 m^2.
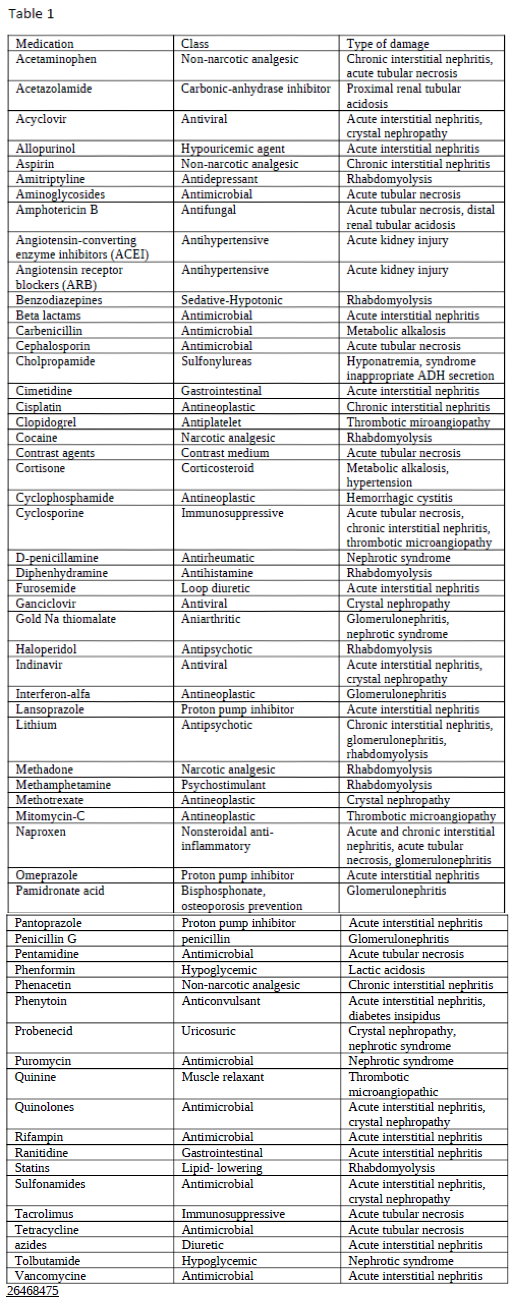
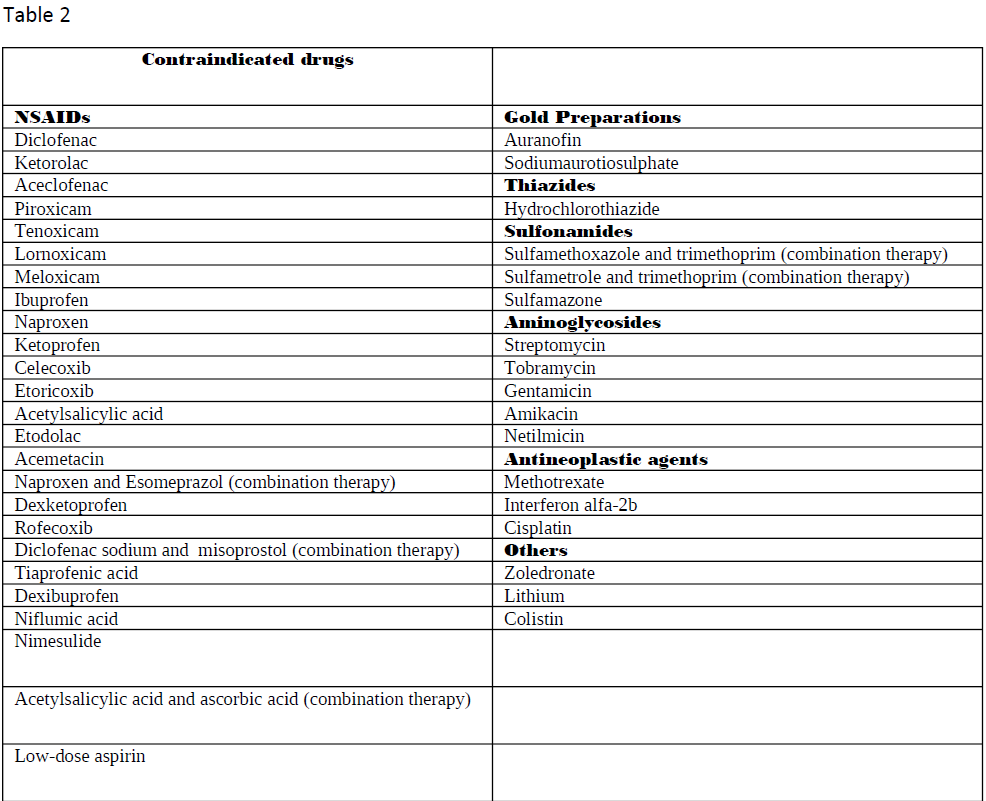
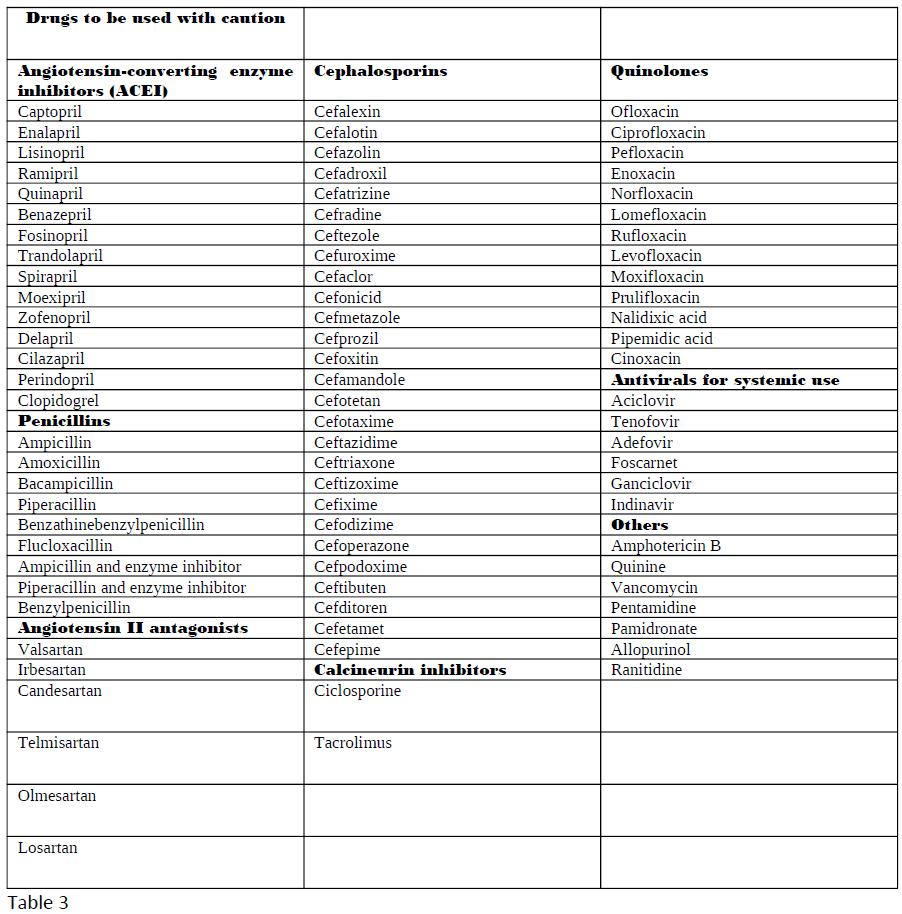
Nephrotoxic medications can elicit damage to the kidney via various mechanisms, including alteration in its structure and function. When evaluating the primary etiologies in renal injury, the incidence of drug-induced toxicity has accounted for 20% of all-cause incidents. Besides, when nephrotoxicity from pharmacologic agents is further examined, evidence has shown it to be one of the chief etiologies in intrinsic renal failure aside from the second most common cause, infection.[1] Numerous drugs go under the category of nephrotoxic medications. Tables 1 through 3 list the most common drugs that can damage the kidneys and their drug class.
The more significant percentage of individuals affected by these agents come from the over 70-year-old category. With the aging life expectancy in the United States, we need to choose pharmacologically appropriate agents for our aging population with declining renal functions.
Mechanism of Action
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Mechanism of Action
Drugs such as those listed previously elicit damage to renal tissue via numerous mechanisms. A few of these mechanisms include impairment of perfusion, inflammation induction, free radicals formation, and many other mechanisms.[2][3] A few of the most common mechanisms of action appear below:
GFR Alteration
- A few examples include the effect of ACE inhibitors, ARBs, cyclosporins, NSAIDs, and tacrolimus that affect the intraglomerular hemodynamics via the alteration of the glomerular filtration rate.[4]
Tubular Cell Toxicity
- The presence of certain pharmaceutical agents may damage the cells of the proximal tubules via free radical formation, mitochondrial damage, and transport systems damage. These agents include aminoglycosides, amphotericin B, adefovir, cisplatin, and foscarnet.[5]
Interstitial Nephritis
- NSAIDs and rifampin are common causes of acute interstitial tissue inflammation. Chronic forms result from agents, including analgesics, anticancer drugs, lithium, and calcineurin inhibitors.[6]
Crystal Nephropathy
- Antivirals such as acyclovir and antibiotics such as ampicillin have been common etiologies of insoluble crystal formation within renal tissue.[7]
- A more detailed list is present in tables 1-3.
Administration
The majority of the drugs described are oral formulations, with a few minor ones available in other dosage forms.
Adverse Effects
As adverse reactions are the main subject, the full list of renal damage locations appears in tables 1 and 2. The primary sites include the glomerulus and proximal and distal convoluted renal tubules.
Contraindications
Contraindications to drug administration include the presence of chronic renal disease or end-stage renal disease.[8] As previously mentioned, the presence of a GFR below 60 ml/min per 1.73 m^2 for the definition of moderate chronic renal disease and a creatinine clearance below 30 mL/min as seen in severe chronic kidney disease are critical metrics for treatment judgment.
Monitoring
General guidelines recommend determining baseline renal function before and after initiation of nephrotoxic pharmaceutical agents. The most common formula used in the calculation of glomerular filtration rate is the Schwartz formula. Calculation of GFR (mL/min/1.73 m^2= length (cm) X potassium /serum creatinine (mg/dL). To use clinician judgment in determining whether to withdraw a nephrotoxic agent, we aim to keep the GFR above the chronic kidney disease threshold of 60.[2]
Also, many biomarkers have become available to assess renal toxicity further. A few of these are as follows:
Urine Proteins and Enzymatic activity
- Alanine aminopeptidase, alpha-glutathione-S-transferase, γ-glutamyl transpeptidase, alkaline phosphatase, N-acetyl-D-glucosaminidase, and π-glutathione-S-transferase 18294749.
Proteinuria
- An increase in the amount of high or low molecular proteins in urine testing is another key diagnostic tool. Typically high and low molecular weight proteins get reabsorbed before being released into the proximal convoluted tubule of the nephron. High molecular weight proteins that signal renal damage include albumin, transferrin, and immunoglobulin G. Low molecular proteins diagnostic for renal damage include a1-microglobulin, B2-microglobulin, cystatin-C, and retinol-binding protein.[9]
Kidney injury Molecule 1
- KIM1 is a type 1 glycoprotein rapidly detected in urine upon damage to tissue that allows for the measurement of proximal renal tubule damage. Greater sensitivity than traditional methods of measuring renal damage such as BUN, Creatinine, and the presence of proteinuria.[5][10]
Neutrophil Gelatinase-associated Lipocalin
- NGAL is synthesized during granulocyte maturation and as a byproduct of tissue inflammation and damage, especially in the proximal renal tubule. Acute kidney injury can be measured and diagnosed earlier with this biomarker. Caution is necessary as NGAL also elevates general inflammatory and infectious processes.
Type 4 Collagen
- Type 4 collagen is present in the basement membrane of the glomerulus. The presence of type 4 collagen in the urine may serve as a critical marker in glomerular membrane damage.[11]
Osteopontin
- Osteopontin is a bone phosphoprotein present in the bone that is useful in measuring damage from certain nephrotoxic drugs. A few of these drugs include cisplatin, angiotensin-2 receptor blockers, cyclosporin, gentamicin, and puromycin.[12]
Clusterin
- Clusterin is a glycoprotein used to measure proximal and distal convoluted tubule damage via presence in the urine.
Preventative measures aimed at decreasing the incidence of significant nephrotoxic effects include oral administration, low effective doses, and short therapy. Furthermore, the dose can be reduced or discontinued in the setting of toxicity.[13]
Toxicity
In the event that there is a significant nephrotoxic event that is changing the GFR, isotonic repletion can be provided to help dilute serum drug concentrations during therapy.[2] Infants and young children are also a chief demographic in drug-induced nephrotoxicity. Toxicity can present with many different types of renal pathology:
- Acute and chronic interstitial nephritis
- Proximal renal tubular acidosis
- Acute and chronic tubular necrosis
- Glomerulonephritis
- Crystal nephropathy
- Nephrotic syndrome
- Rhabdomyolysis
Understanding the different regions of renal damage may allow for a more targeted approach to renal injury, including the development of pharmaceutical agents with greater benefit and decreased toxicity.[14][15]
Enhancing Healthcare Team Outcomes
Understanding nephrotoxic pharmaceutical agents is a key component of the interprofessional healthcare team and a patient-centered approach. Starting with the clinicians who prescribe the drugs to the pharmacists who dispense the drug and the nurses who administer the drugs, there is a chain of transport where many healthcare providers are a part of patient care. Knowing the toxic effects, these agents may significantly impact the quality of care a patient receives. The nurse should be aware of which drugs are nephrotoxic, especially with patients that already have renal impairment. They can counsel the patients regarding these agents and report any issues back to the prescriber. The pharmacist can reinforce this counsel while also performing total medication reconciliation, checking doses (especially those that require renal adjustment), and contacting the clinician if there are any issues. These types of interprofessional collaborations will prevent renal toxicity from occurring when it need not. [Level 5]
Furthermore, through the understanding of preventative measures and thresholds for toxicity, healthcare providers may enhance patient safety. Lastly, with the development of numerous biomarkers targeting types of renal damage, response to treatment can be monitored when knowledge of the potential renal effects of medication is known. [Level 1] [14][16]
Media
References
Kaufman J, Dhakal M, Patel B, Hamburger R. Community-acquired acute renal failure. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1991 Feb:17(2):191-8 [PubMed PMID: 1992662]
Ghane Shahrbaf F, Assadi F. Drug-induced renal disorders. Journal of renal injury prevention. 2015:4(3):57-60. doi: 10.12861/jrip.2015.12. Epub 2015 Sep 1 [PubMed PMID: 26468475]
Schetz M, Dasta J, Goldstein S, Golper T. Drug-induced acute kidney injury. Current opinion in critical care. 2005 Dec:11(6):555-65 [PubMed PMID: 16292059]
Level 3 (low-level) evidencePerazella MA. Drug-induced nephropathy: an update. Expert opinion on drug safety. 2005 Jul:4(4):689-706 [PubMed PMID: 16011448]
Level 3 (low-level) evidenceMarkowitz GS, Fine PL, Stack JI, Kunis CL, Radhakrishnan J, Palecki W, Park J, Nasr SH, Hoh S, Siegel DS, D'Agati VD. Toxic acute tubular necrosis following treatment with zoledronate (Zometa). Kidney international. 2003 Jul:64(1):281-9 [PubMed PMID: 12787420]
Level 3 (low-level) evidencePerneger TV, Whelton PK, Klag MJ. Risk of kidney failure associated with the use of acetaminophen, aspirin, and nonsteroidal antiinflammatory drugs. The New England journal of medicine. 1994 Dec 22:331(25):1675-9 [PubMed PMID: 7969358]
Level 2 (mid-level) evidenceMarkowitz GS, Perazella MA. Drug-induced renal failure: a focus on tubulointerstitial disease. Clinica chimica acta; international journal of clinical chemistry. 2005 Jan:351(1-2):31-47 [PubMed PMID: 15563870]
Level 3 (low-level) evidenceIngrasciotta Y, Sultana J, Giorgianni F, Caputi AP, Arcoraci V, Tari DU, Linguiti C, Perrotta M, Nucita A, Pellegrini F, Fontana A, Cavagna L, Santoro D, Trifirò G. The burden of nephrotoxic drug prescriptions in patients with chronic kidney disease: a retrospective population-based study in Southern Italy. PloS one. 2014:9(2):e89072. doi: 10.1371/journal.pone.0089072. Epub 2014 Feb 18 [PubMed PMID: 24558471]
Level 2 (mid-level) evidenceFerguson MA, Vaidya VS, Bonventre JV. Biomarkers of nephrotoxic acute kidney injury. Toxicology. 2008 Mar 20:245(3):182-93. doi: 10.1016/j.tox.2007.12.024. Epub 2008 Jan 4 [PubMed PMID: 18294749]
Level 3 (low-level) evidenceVaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. Annual review of pharmacology and toxicology. 2008:48():463-93 [PubMed PMID: 17937594]
Level 3 (low-level) evidenceNerlich AG, Schleicher ED, Wiest I, Specks U, Timpl R. Immunohistochemical localization of collagen VI in diabetic glomeruli. Kidney international. 1994 Jun:45(6):1648-56 [PubMed PMID: 7933812]
Alchi B, Nishi S, Kondo D, Kaneko Y, Matsuki A, Imai N, Ueno M, Iguchi S, Sakatsume M, Narita I, Yamamoto T, Gejyo F. Osteopontin expression in acute renal allograft rejection. Kidney international. 2005 Mar:67(3):886-96 [PubMed PMID: 15698428]
Guo X, Nzerue C. How to prevent, recognize, and treat drug-induced nephrotoxicity. Cleveland Clinic journal of medicine. 2002 Apr:69(4):289-90, 293-4, 296-7 passim [PubMed PMID: 11996200]
Kim SY, Moon A. Drug-induced nephrotoxicity and its biomarkers. Biomolecules & therapeutics. 2012 May:20(3):268-72. doi: 10.4062/biomolther.2012.20.3.268. Epub [PubMed PMID: 24130922]
Choudhury D, Ahmed Z. Drug-associated renal dysfunction and injury. Nature clinical practice. Nephrology. 2006 Feb:2(2):80-91 [PubMed PMID: 16932399]
Bonventre JV, Vaidya VS, Schmouder R, Feig P, Dieterle F. Next-generation biomarkers for detecting kidney toxicity. Nature biotechnology. 2010 May:28(5):436-40. doi: 10.1038/nbt0510-436. Epub [PubMed PMID: 20458311]
Level 3 (low-level) evidence