Introduction
Mohs micrographic surgery is a tissue-sparing, precise method of skin cancer removal named in honor of the surgeon who developed the technique, Frederick Mohs. It is a surgical approach that offers high cure rates for the treatment of a variety of skin cancers, including basal cell carcinomas (BCC) and squamous cell carcinomas (SCC). The main advantage of Mohs surgery is that it offers precise microscopic control of the entire tumor margin while maximizing conservation of healthy tissue.
This technique was developed by Dr. Mohs in the 1930’s. The procedure was originally named “chemosurgery,” since the technique involved the application of a chemical fixative (zinc chloride) to the in-situ tumor. After 24 hours of in-situ fixation, the tumor was excised and microscopically examined. The process was repeated until the tumor was completely removed.[1][2] Over the following decades, Mohs surgery shifted away from using zinc chloride fixation in favor of processing fresh tissue that was frozen and sectioned in a cryostat microtome. This technique offered several advantages compared to the original chemosurgery technique, including faster processing times (15 to 30 minutes), decreased patient discomfort, and increased tissue conservation.[3]
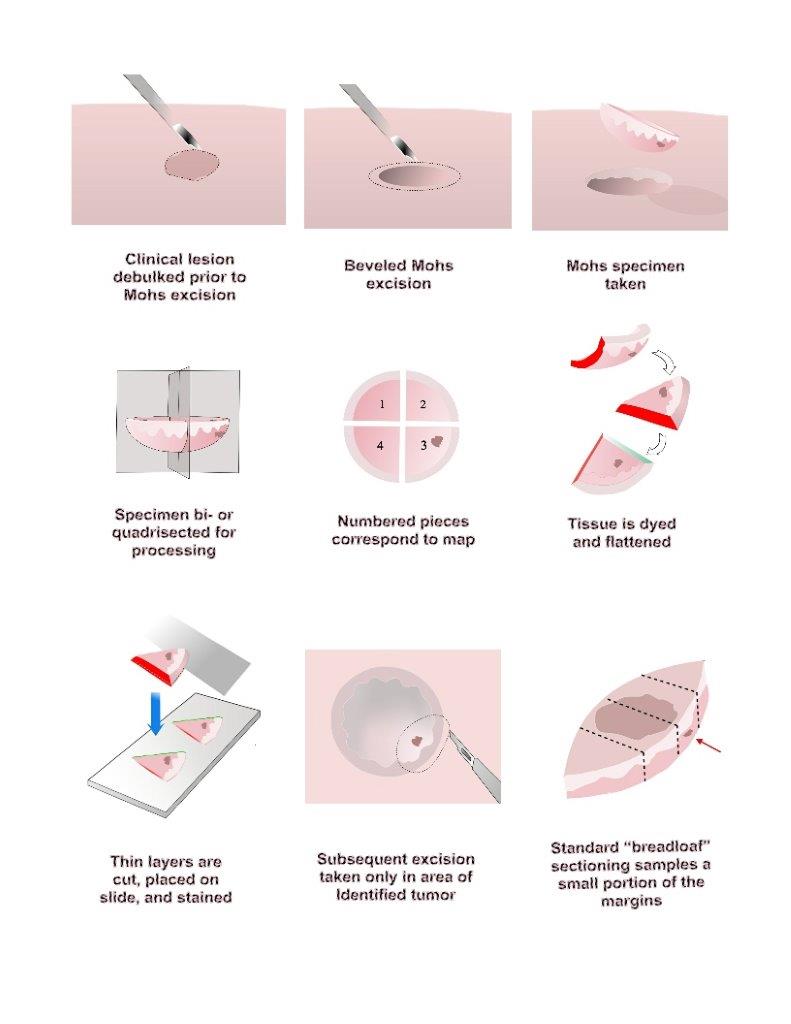
Mohs surgery is appropriate for skin cancers with a high risk of recurrence and when tissue conservation is essential. [4][5] It is performed by removing a thin margin of tissue circumferentially around and deep to the clinical margins of a skin tumor. The specimen is typically removed with a 45-degree bevel to facilitate tissue processing. It is then rapidly frozen and sectioned in a cryostat microtome, allowing for quick tissue processing (about 15 to 30 minutes). Sectioning the tissue in a horizontal direction allows virtually 100% of the tissue margin (peripheral and deep margins) to be examined under the microscope. The process is repeated until the tumor has negative histologic margins.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
The tissue-sparing properties of Mohs micrographic surgery make it particularly useful in areas of functional and aesthetic importance such as the head and neck area, anogenital area, hands, and feet.
Indications
Mohs surgery is appropriate for skin cancers with a high risk of recurrence and when tissue conservation is essential. The Mohs Appropriate Use Criteria (AUC) guidelines were developed to assist clinicians in determining if a specific tumor would be appropriately managed by Mohs surgery. A Mohs AUC mobile phone app is available for download to mobile devices. These criteria were based on areas of the body, patient characteristics, and tumor characteristics.[5]
Mohs surgery is particularly suitable for areas of the body in the "H" area:
- Central face, eyelids/canthi, eyebrows, nose, lips, chin, ear, and periauricular area
- Genitalia
- Hands, feet, ankles, and nail units
- Nipples/areola
Higher-risk patient characteristics include:
- Immunocompromised
- Genetic syndromes (basal cell nevus syndrome, xeroderma pigmentosum)
- Prior radiated skin
- Patient with history of high-risk tumors
Tumor characteristics include:
- Positive margin on recent excision
Aggressive features that are high risk for recurrence of BCC:
- Aggressive histologic subtype: morpheaform, infiltrating, micronodular
- Perineural involvement
- Metatypical/keratotic
Aggressive features of SCC:
- Poorly or undifferentiated (characterized by a high degree of nuclear polymorphism, high mitotic rate, or low degree of keratinization)
- Perineural/perivascular
- Spindle cell
- Breslow depth 2 mm or greater
- Clark level IV or greater
While the Mohs AUC can be helpful in determining if a specific lesion is appropriately managed with Mohs surgery, it does not exclude the validity of alternate modalities in treating the same lesion (e.g. curettage, electrodesiccation & curettage, or excision). [4][5]
Contraindications
There are no absolute contraindications to Mohs surgery in patients deemed suitable for surgery in general.
Equipment
Mohs micrographic surgery requires equipment for the operating room as well as for the lab in which tissue is processed and examined microscopically. The operating room requires good lighting and an adjustable table to provide optimal visualization and access to the tumor. Surgical equipment is relatively simple, consisting of a scalpel, fine forceps, scissors, gauze, and an electrosurgical device for coagulation. Reconstruction can be achieved with an expanded tray that includes needle holders, scissors, fine forceps, skin hooks, and a scalpel.
The Mohs histology laboratory consists of microtomes that freeze tissue and then allow cutting of very thin slices of tissue to mount on glass slides. The slides are then placed in an automated stainer or may be stained by hand. This process may require a vent hood to minimize exposure to chemicals involved in the staining process. Completed slides are then read by the Mohs surgeon under light microscopy to determine if tumor remains in the tissue. Many Mohs labs also have special stainers and reagents to allow immunohistochemical staining of tissue.
Personnel
The procedure requires the surgeon and at least one assistant in the surgical suite. In addition, at least one histotechnician is needed in the Mohs laboratory for tissue processing.
Technique or Treatment
The technique of Mohs surgery is as follows:
- The tumor is first outlined prior to injection with a local anesthetic. After anesthetized, any visible tumor is removed or “debulked,” with a curette, flexible blade, or scalpel.
- Prior to removal, the tissue layer is carefully oriented by placing small superficial etch marks with a scalpel (often at 3 o’clock, 6 o’clock, 9 o’clock, and noon) around the tissue layer and corresponding in-situ skin.
- A thin margin of tissue is then removed circumferentially around and deep to the debulked tumor defect. This “layer” of tissue is removed with a beveled angle of approximately 45 degrees, which facilitates tissue processing (see below).
- Once removed, the tissue layer is often cut into halves or quadrants and then marked with colored dyes to facilitate precise mapping of the tumor. The tissue is then pressed flat, so the epidermal edge occupies the same tissue plane as the deep margin. The “beveled” edge acquired tissue removal facilitates this flattening process.
- The tissue is then cut and processed in a horizontal direction so that virtually 100% of the peripheral and deep margin can be examined on the same tissue section under the microscope. This is in contrast to the traditional vertical, or “breadloafed,” tissue processing which examines only a small portion of the tumor margin.[6]
- If residual tumor is identified under the microscope, then the Mohs map is marked and the corresponding in-situ tissue is precisely removed from the patient in that portion that was found to still have tumor. This process is repeated until the tumor is histologically negative, thus ensuring complete tumor removal with maximum conservation of healthy tissue.
- Once the tumor has been removed, a variety of techniques are used to close the defect, including primary closure, flaps, grafts, and second intention healing. A recent tabulation of Mohs stages per case for experienced Mohs surgeons showed a median of about 1.7 stages per tumor to clear. Obviously, that number can be much higher for more complicated cases.[7]
Tissue stains most commonly used for Mohs surgery are hematoxylin and eosin (H&E) and toluidine blue. While the majority of Mohs surgeons use H&E routinely, a significant minority prefer toluidine blue for processing basal cell carcinoma, since mucopolysaccharides and hyaluronic acid that are associated with BCC stain metachromatically with a magenta coloration.[8][9][10]
The Mohs procedure depends upon the presence of continuous tumor growth (no "skip" areas) to be maximally effective. Fortunately, this characteristic is present in most cancers that occur on the skin.
Clinical Significance
Mohs surgery has had a high degree of clinical success.
- Mohs surgery reports excellent 5-year cure rates for non-melanoma skin cancers (NMSC), in particular basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). Examples of 5-year cure rates include: Primary BCC (99%), recurrent BCC (94.4%), primary SCC (92-99%), and recurrent SCC (90%). [11][12][13]
- Mohs surgery also can be used to treat other less common tumors, including dermatofibrosarcoma protuberans, microcystic adnexal carcinoma, extramammary Paget disease, Merkel cell carcinoma, and sebaceous carcinoma.[14] More recently, with the availability of reliable immunohistochemical stains, Mohs micrographic has also shown great usefulness in treating some forms of malignant melanoma, including lentigo maligna, lentigo maligna melanoma, and thin melanomas. [15]
Enhancing Healthcare Team Outcomes
The procedure requires the operative surgeon and nurse to work together in the surgical suite. In addition, at least one histotechnician is needed in the Mohs laboratory for tissue processing. A coordinated team approach provides the best results for patient care. [Level V]
Media
(Click Image to Enlarge)
References
Mohs FE. Chemosurgery. Clinics in plastic surgery. 1980 Jul:7(3):349-60 [PubMed PMID: 7438703]
Mohs FE. Chemosurgery for the microscopically controlled excision of cutaneous cancer. Head & neck surgery. 1978 Nov-Dec:1(2):150-66 [PubMed PMID: 755808]
Tromovitch TA, Stegman SJ. Microscopie-controlled excision of cutaneous tumors: chemosurgery, fresh tissue technique. Cancer. 1978 Feb:41(2):653-8 [PubMed PMID: 75761]
Asgari MM, Olson JM, Alam M. Needs assessment for Mohs micrographic surgery. Dermatologic clinics. 2012 Jan:30(1):167-75, x. doi: 10.1016/j.det.2011.08.010. Epub [PubMed PMID: 22117877]
Ad Hoc Task Force, Connolly SM, Baker DR, Coldiron BM, Fazio MJ, Storrs PA, Vidimos AT, Zalla MJ, Brewer JD, Smith Begolka W, Ratings Panel, Berger TG, Bigby M, Bolognia JL, Brodland DG, Collins S, Cronin TA Jr, Dahl MV, Grant-Kels JM, Hanke CW, Hruza GJ, James WD, Lober CW, McBurney EI, Norton SA, Roenigk RK, Wheeland RG, Wisco OJ. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. Journal of the American Academy of Dermatology. 2012 Oct:67(4):531-50. doi: 10.1016/j.jaad.2012.06.009. Epub 2012 Sep 5 [PubMed PMID: 22959232]
Rapini RP. Comparison of methods for checking surgical margins. Journal of the American Academy of Dermatology. 1990 Aug:23(2 Pt 1):288-94 [PubMed PMID: 2212126]
Krishnan A, Xu T, Hutfless S, Park A, Stasko T, Vidimos AT, Leshin B, Coldiron BM, Bennett RG, Marks VJ, Brandt R, Makary MA, Albertini JG, the American College of Mohs Surgery Improving Wisely Study Group. Outlier Practice Patterns in Mohs Micrographic Surgery: Defining the Problem and a Proposed Solution. JAMA dermatology. 2017 Jun 1:153(6):565-570. doi: 10.1001/jamadermatol.2017.1450. Epub [PubMed PMID: 28453605]
Todd MM, Lee JW, Marks VJ. Rapid toluidine blue stain for Mohs' micrographic surgery. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2005 Feb:31(2):244-5 [PubMed PMID: 15762224]
Humphreys TR, Nemeth A, McCrevey S, Baer SC, Goldberg LH. A pilot study comparing toluidine blue and hematoxylin and eosin staining of basal cell and squamous cell carcinoma during Mohs surgery. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 1996 Aug:22(8):693-7 [PubMed PMID: 8780761]
Level 3 (low-level) evidenceGoldberg LH, Wang SQ, Kimyai-Asadi A. The setting sun sign: visualizing the margins of a basal cell carcinoma on serial frozen sections stained with toluidine blue. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2007 Jun:33(6):761-3 [PubMed PMID: 17550460]
Level 3 (low-level) evidenceRowe DE, Carroll RJ, Day CL Jr. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. The Journal of dermatologic surgery and oncology. 1989 Mar:15(3):315-28 [PubMed PMID: 2646336]
Roenigk RK, Roenigk HH Jr. Current surgical management of skin cancer in dermatology. The Journal of dermatologic surgery and oncology. 1990 Feb:16(2):136-51 [PubMed PMID: 2406310]
Rowe DE, Carroll RJ, Day CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. Journal of the American Academy of Dermatology. 1992 Jun:26(6):976-90 [PubMed PMID: 1607418]
Thomas CJ, Wood GC, Marks VJ. Mohs micrographic surgery in the treatment of rare aggressive cutaneous tumors: the Geisinger experience. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2007 Mar:33(3):333-9 [PubMed PMID: 17338692]
Level 2 (mid-level) evidenceEtzkorn JR, Sobanko JF, Elenitsas R, Newman JG, Goldbach H, Shin TM, Miller CJ. Low recurrence rates for in situ and invasive melanomas using Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining: tissue processing methodology to optimize pathologic staging and margin assessment. Journal of the American Academy of Dermatology. 2015 May:72(5):840-50. doi: 10.1016/j.jaad.2015.01.007. Epub 2015 Mar 13 [PubMed PMID: 25774012]
Level 2 (mid-level) evidence