 Neuroanatomy, Medial Lemniscus (Reils Band, Reils Ribbon)
Neuroanatomy, Medial Lemniscus (Reils Band, Reils Ribbon)
Introduction
The medial lemniscus is a second-order neuron of the dorsal column-medial lemniscus pathway (DCML), which, with the somatotopic arrangement, transports the sensory spinothalamic information of conscious proprioception, vibration, fine touch, and 2-point discrimination of skin and joints of the body and head; from the caudal medulla to the ventral posterolateral nucleus of the thalamus, and subsequently the primary somatosensory cortex. The medial lemniscus (second-order neuron of DCML) commences at the nucleus gracilis and nucleus cuneatus at the caudal medulla; the arcuate fibers decussate at the caudal medulla and ascend via the medial lemniscus contralaterally in the brainstem until synapsing at the ventral posterolateral nucleus of the thalamus, the point at which the third-order neuron of the DCML pathway commences. The third-order neuron ascends from the ventral posterolateral nucleus until synapsing with the primary somatosensory region of the brain cortex. Due to the unique somatosensory information and location of this pathway, the dorsal column-medial lemniscus tract may be used as an important clinical clue to pinpoint a possible site of injury and manage the patient accordingly.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
The primary function of the medial lemniscus is as a second-order neuron of the dorsal column-medial lemniscus pathway (DCML) is to transport the sensory spinothalamic information of conscious proprioception, vibration, fine touch, and 2-point discrimination of skin and joints of the body and head; from the caudal medulla to the ventral posterolateral nucleus of the thalamus, and subsequently the primary somatosensory cortex.
Embryology
The embryological origin of the medial lemniscus, just like the entire nervous system is of neural ectoderm origin. The neural ectoderm further divides into three main sub-divisions that contribute to the DCML pathway.
The neural plate, responsible for the central nervous system (CNS) develops cerebral vesicles, including[1]:
- The myelencephalon, which develops into the medulla oblongata
- The metencephalon developing into the pons
- The mesencephalon developing into the midbrain
- The diencephalon developing into the thalamus
- The telencephalon developing into the cerebral cortex
As previously described, all of these structures are important components of the DCML tract and its development. Furthermore, the protein Netrin-1 secreted from the floor plate of the medulla oblongata plays an important role in the development and migration of the axons forming the medial lemniscus.[2]
The neural crest cells responsible for the peripheral nervous system (PNS), including the somatic sensory nerves, give rise to first-order neurons of the DCML pathway. It contributes to the development of tactile mechanoreceptors such as Meissner corpuscles giving light touch and vibrations, Ruffini corpuscles detecting tension in the skin, Merkel discs detecting pressure, and Pacinian corpuscles detecting high-frequency vibration.[3]
Lastly, the ectodermal placodes, which provide cranial sensory ganglia, develop into the chief sensory nucleus of the trigeminal nerve whose axons also join the medial lemniscus pathway to provide conscious proprioception, vibration, fine touch, and 2-point discrimination of the head.
Blood Supply and Lymphatics
The blood supply of the medial lemniscus varies according to the level of the brainstem. The nuclei gracilis and cuneatus are located in the medial posterior aspect of the medulla and receive vascular supply from the posterior spinal artery. The arcuate fibers decussate in the medial-anterior aspect of the medulla and are irrigated predominantly by the anterior spinal artery. The medial lemniscus begins ascending contralaterally in the brainstem in the medial aspect of the medulla and is supplied by the anterior spinal artery. As the medial lemniscus continues to ascend, it begins shifting posteriorly and laterally; at the level of the pons, the medial lemniscus receives supply from the basilar artery. By the time the medial lemniscus makes it to the midbrain, it is predominantly in the posterior lateral aspect of the midbrain, and the posterior cerebral artery irrigates it.[4]
Infarction of certain regions of the brainstem may present with loss of conscious proprioception, vibration, fine touch, and 2-point discrimination due to the medial lemniscus being at different positions of the brainstem, and thalamus.
Surgical Considerations
The medial lemniscus (second-order neuron of the DCML pathway) is mostly contained within the brainstem. Due to the high density of neurological tracts and nuclei in the brainstem, it is complex to perform surgery targeting specific pathways within it. Surgery in the brainstem may result in additional neurologic tracts suffering damage; therefore, surgery has historically been reserved for clear-cut cases in which benefits outweigh risks. Researchers conducted a prospective randomized clinical trial, in which motor deficit and neurological status outcomes underwent evaluation using preoperative diffusion tensor imaging (DTI) and diffusion tensor tractography (DTT) in patients undergoing surgery for cavernous brainstem malformations. Although it is too early to make any conclusions, results seem promising as specific tracts and pathologies may be visualized, allowing for a better surgical approach, decision making, and potentially decreasing morbidities.[5][6]
Clinical Significance
The DCML pathway may be affected at numerous points throughout its first, second, and third-order neurons. The clinical presentation may be convoluted due to its proximity to other neurologic tracts; furthermore, the absence of conscious proprioception, vibration, fine touch, and 2-point discrimination of skin and joints is pathognomonic for damage of the DCML pathway. The level at which the DCML pathway is affected will determine the clinical presentation. Below is a list of pathologies in which the DCML pathway is affected.
-
Tabes dorsalis: This pathology results from the spirochete Treponema pallidum in advanced tertiary syphilis. Although rarely seen due to T. pallidum sensitivity to penicillin, tabes dorsalis results in demyelination of the dorsal column and roots, typically affecting the lower limbs due to the lumbosacral region being the one that is predominantly affected. The lack of conscious proprioception, vibration, fine touch, and 2-point discrimination of skin and joints results in neuropathic osteoarthropathy (Charcot joint). The Charcot joint presents with progressive degeneration of weight-bearing joints; the most common joint affected is midfoot. Accompanying complications include bone fractures and ulcerations of the site of injury. Management of this condition is targeting the underlying infectious agent with intravenous penicillin.[7]
-
Vitamin B12 deficiency: Subacute, combined degeneration is the demyelination of the dorsal columns, lateral corticospinal tracts, and spinocerebellar tracts due to vitamin B12 deficiency. Vitamin B12 is rarely deficient in the United States; however, one must always rule it out in specific patient groups at higher risk such as strict vegans, patients suffering from inflammatory bowel disease (IBD), chronic malabsorption, pernicious anemia, history of gastrectomy, diphyllobothrium latum infection, and cystic fibrosis. The condition is diagnosable by obtaining methylmalonic acid levels. The treatment is vitamin B12 supplementation; however, the extent of recovery will be dependent on the duration and extent of the neuronal damage.[8]
-
Brown-Sequard syndrome: This pathology is a theoretical injury that is rarely seen in practice as clear cut as described in medical textbooks. It is composed of a hemisection of the spinal cord, typically due to trauma, ischemia, a large unilateral tumor, or infectious agents. The hemisection results in loss of the ipsilateral DCML pathway, ipsilateral corticospinal tract damage, contralateral spinothalamic tract damage; also, it results in flaccid paralysis and loss of all sensation at the level of injury. The clinical presentation is with loss of conscious proprioception, vibration, fine touch, and 2-point discrimination of skin and joints on the ipsilateral side of injury, spasticity, and hyperreflexia on the ipsilateral side of injury, loss of pain and temperature in the contralateral side of injury, and flaccid paralysis and loss of all sensation at the level of injury. Diagnosis is possible through magnetic resonance imaging, and a neurologic exam detailing a clinical presentation similar to those mentioned above. Treatment is specific to the underlying pathology.[9]
-
Medial medullary syndrome: Medial medullary syndrome is an infarction of the medial aspect of the medulla oblongata due to occlusion of the paramedian branches of the anterior spinal artery. The infarction results in loss of conscious proprioception, vibration, fine touch, and 2-point discrimination of skin and joints of the contralateral side due to damage to the medial lemniscus. Also, it presents with contralateral hemiparesis due to damage to the corticospinal tract before decussation at the medullary pyramids and ipsilateral tongue deviation due to hypoglossal nerve fibers damage. The evaluation includes coagulation studies, echocardiogram, and CT angiography. Management is dependent on the time of onset, and type of stroke (ischemic versus hemorrhagic).
-
Paramedian artery occlusion: The paramedian arteries are a branch of the basilar artery that supplies oxygenated blood to the pons. Paramedian artery occlusion is also a condition that rarely presents clinically. However, it is essential to point out that the paramedian arteries irrigate the medial basal pons, pontine nuclei, corticospinal fibers, and the medial lemniscus as it ascends through the pons. The evaluation includes coagulation studies, echocardiogram, and CT angiography. Management is dependent on the time of onset, and type of stroke (ischemic versus hemorrhagic).
-
Upper dorsal pontine syndrome: This syndrome is also known as “Raymond Cestan syndrome” and is due to occlusion of the long circumferential branches of the basilar artery. The occlusion of the results mentioned above in damage to the medial lemniscus pathway, resulting in contralateral loss of conscious proprioception, vibration, fine touch, and 2-point discrimination of skin and joints. Furthermore, it may present with ipsilateral ataxia, ipsilateral paralysis to the muscles of mastication, ipsilateral sensory loss of the face, horizontal gaze palsy, contralateral loss of touch pain and temperature, and contralateral hemiparesis of the face and body. The evaluation includes coagulation studies, echocardiogram, and computed tomogram (CT) angiography. Management is dependent on the time of onset, and type of stroke (ischemic versus hemorrhagic).[10]
In addition to the syndromes above, the following may present with damage to the medial lemniscus pathway:
-
Dorsal midbrain syndrome due to occlusion of the paramedian branches of the basilar artery may result in medial lemniscus damage if the occlusion has a lateral extension.
-
Thalamic strokes causing damage to the ventral posterolateral nucleus due to occlusion of thalamic branches of the posterior cerebral artery could result in damage to the third-order neuron of the DCML pathway.
Media
(Click Image to Enlarge)
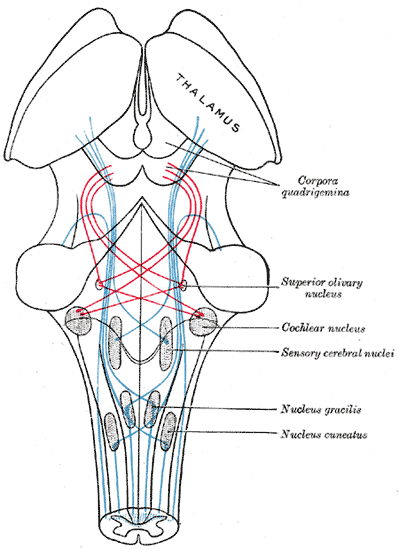
The Midbrain or Mesencephalon, the Course of the Fibers of the Lemniscus. The illustration shows the medial lemniscus in blue, lateral in red, thalamus, corpora quadrigemina, superior olivary nucleus, cochlear nucleus, sensory cerebral nuclei, nucleus gracilis, and nucleus cuneatus.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
References
Elshazzly M, Lopez MJ, Reddy V, Caban O. Embryology, Central Nervous System. StatPearls. 2025 Jan:(): [PubMed PMID: 30252280]
Kubota C, Nagano T, Baba H, Sato M. Netrin-1 is crucial for the establishment of the dorsal column-medial lemniscal system. Journal of neurochemistry. 2004 Jun:89(6):1547-54 [PubMed PMID: 15189358]
Level 3 (low-level) evidencePiccinin MA, Miao JH, Schwartz J. Histology, Meissner Corpuscle. StatPearls. 2025 Jan:(): [PubMed PMID: 30085522]
Romanowski CA, Hutton M, Rowe J, Yianni J, Warren D, Bigley J, Wilkinson ID. The Anatomy of the Medial Lemniscus within the Brainstem Demonstrated at 3 Tesla with High Resolution Fat Suppressed T1-Weighted Images and Diffusion Tensor Imaging. The neuroradiology journal. 2011 May 15:24(2):171-6 [PubMed PMID: 24059604]
Li D, Jiao YM, Wang L, Lin FX, Wu J, Tong XZ, Wang S, Cao Y. Surgical outcome of motor deficits and neurological status in brainstem cavernous malformations based on preoperative diffusion tensor imaging: a prospective randomized clinical trial. Journal of neurosurgery. 2019 Jan 1:130(1):286-301. doi: 10.3171/2017.8.JNS17854. Epub 2018 Mar 16 [PubMed PMID: 29547081]
Level 1 (high-level) evidenceRanzenberger LR, Das JM, Snyder T. Diffusion Tensor Imaging. StatPearls. 2025 Jan:(): [PubMed PMID: 30726046]
Bhandari J, Thada PK, Leslie SW, Ratzan RM. Tabes Dorsalis. StatPearls. 2025 Jan:(): [PubMed PMID: 32491814]
Durrani MI, Basit H, Blazar E. Diphyllobothriasis (Fish Tapeworm Infection). StatPearls. 2025 Jan:(): [PubMed PMID: 31082015]
Shams S, Davidson CL, Arain A. Brown-Séquard Syndrome. StatPearls. 2025 Jan:(): [PubMed PMID: 30844162]
Krasnianski M, Wendt M, Müller T, Zierz S. Raymond-Cestan syndrome in pontine ischemia. Clinical neurology and neurosurgery. 2007 Nov:109(9):834-5 [PubMed PMID: 17698284]
Level 3 (low-level) evidence