Introduction
Chronic pain is a complex condition characterized by changes in the nervous system and clinical manifestations influenced by psychosocial and potentially iatrogenic factors.[1] Repeated activation of the nociceptive system abnormally sensitizes the peripheral and central neural pathways that typically convert noxious environmental stimuli into perceived pain in the brain.[2] These abnormal pathways can present clinically as severe, persistent, and functionally disabling pain, which is often challenging to manage despite individualized care with oral medications and psychosocial treatments. Attaining good outcomes for patients with severe chronic pain has recently become even more difficult because of the critical safety concerns and public health issues associated with prescription opioids.[3]
Advanced pain management options should be considered when patients cannot reach their analgesic and functional goals with more conventional therapies. One advanced interventional modality involves delivering analgesics directly into the cerebrospinal fluid (CSF) via pump and catheter. The first pump providing low-dose morphine in a patient with cancer-related pain was implanted in 1981. Since then, several systematic reviews have demonstrated intrathecal drug delivery systems to be effective and safe for cancer-related and chronic noncancer pain.[4]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Administering drugs via oral or parenteral routes often encounters obstacles due to the blood-brain barrier, which acts as a protective barrier preventing many substances, especially macromolecules, from effectively reaching the central nervous system. Intrathecal therapy circumvents this obstacle by directly administering drugs into the CSF, enabling them to reach target sites such as the dorsal horn of the spinal cord without being impeded by the blood-brain barrier or undergoing first-pass metabolism. Intrathecal therapy permits lower overall dosages and reduces the likelihood of systemic adverse events due to minimal interaction with systemic receptors.
An intrathecal drug delivery system consists of a pump and an intrathecal catheter. The pump serves as the drug reservoir, and it is implanted under the subcutaneous tissue in the abdominal area. The catheter attaches to the pump and delivers medications directly into the intrathecal space. Generally, catheter tip placement is at the spinal cord level, innervating the body region of the primary pain source.[5] Once in the CSF, intrathecally delivered medications permeate across the pia mater, arachnoid, and white matter of the spinal cord to access the target dorsal horn receptors and ion channels engaged in nociceptive processing and transmission.[6]
To date, 3 medications have been specifically authorized by the United States Food and Drug Administration (FDA) for intrathecal delivery. Morphine is a mu-opioid receptor agonist that utilizes its spinal effects by binding to receptors in the substantia gelatinosa in the dorsal column. Presynaptic and postsynaptic receptor activation with morphine yields dampening of ascending pain-related neural activity. The second FDA-approved intrathecal analgesic is ziconotide, a potent drug derived from the neurotoxic venom of cone snails. Ziconotide exerts its analgesic effect by reversibly antagonizing the presynaptic N-type voltage-gated calcium channels expressed by nociceptive neurons in the dorsal horn.[7] The third approved medication is baclofen, which is used to treat muscle spasticity from a variety of conditions such as multiple sclerosis and cerebral palsy.
Indications
The indications for placing an intrathecal drug delivery system are cancer-related pain, noncancer pain conditions, and severe spasticity. Patients with cancer and cancer-related pain refractory to analgesic medications delivered via other routes, such as orally or transdermally, may benefit from intrathecal drug delivery. Patients with noncancer hard-to-manage pain conditions such as phantom limb pain, complex regional pain syndrome type I (formerly reflex sympathetic dystrophy), complex regional pain syndrome type II (formerly causalgia), and plexopathies, including brachial, lumbosacral, or radiation-induced, may also benefit from intrathecal drug delivery. Additionally, patients who have refractory pain secondary to failed interventional or surgical techniques, including those with failed back surgery syndrome or lumbar postlaminectomy syndrome, carry an indication for intrathecal drug delivery. Intrathecal administration of baclofen is indicated in patients with serious side effects or limited response to the maximum doses of oral baclofen, tizanidine, or dantrolene.
Pain Management
Persistent chronic pain is frequent in patients with cancer and has a severe impact on their health-related quality of life. A recent systematic review found that 38% of patients with cancer rated their pain as moderate or severe.[8] Furthermore, pain prevalence was 39.3% after curative treatment, 55% during anticancer therapies, and 66.4% in advanced, metastatic, or end-stage disease.[8]
According to the World Health Organization (WHO) guidelines, the management of cancer pain in adolescents and adults typically includes pharmacological and nonpharmacological modalities or a combination thereof.[9] Pharmacological treatments will vary with the type of pain. Managing neuropathic pain frequently requires the solitary or combined use of medications such as acetaminophen, nonsteroidal anti-inflammatory drugs, opioids, antiepileptic drugs, tricyclic antidepressants, muscle relaxants, ketamine, local capsaicin, or cannabinoids. Systemic opioids are the most commonly used agents. Numerous studies indicate that as many as 80% of patients treated with opioids experience at least 1 adverse event, most commonly nausea, vomiting, constipation, or respiratory depression. Long-term effects of opioid therapy can include dependence, tolerance, and immunosuppression.
The efficacy of pharmacologic therapy is often limited in chronic pain conditions, especially those featuring neuropathic pain. Despite full compliance with the WHO analgesia guidelines, pain control remains inadequate for patients with cancer, and a multimodal, interprofessional approach is often the most effective and safest therapy.[10] In this context, the intrathecal route of drug delivery can be an effective option for managing chronic refractory pain. By placing a catheter in the CSF, intrathecal therapy permits the direct delivery of opioid and nonopioid drugs to the receptors of the central nervous system (CNS), reducing adverse effects and systemic dosages.
Spasticity
Spasticity is a motor disorder characterized by muscle tension or rigidity with impaired voluntary movement. Spasticity is a serious concern for many individuals affected by multiple sclerosis, spinal cord injury, cerebral palsy, and acquired brain injuries such as stroke. Increased muscle tone and spasms impair mobility and interfere with the normal activities of daily life. Spasticity can co-occur with significant pain and discomfort, skin rupture, contracture, and sleep disorders. The treatment goals are to reduce spasticity, energy expenditure, and pain and to improve movement, walking, and autonomy in daily activities or rehabilitation therapy. Current treatments for spasticity include physical therapy, oral medications, botulinum toxin injections, or surgery. Alternatively, intrathecal treatment with baclofen is widely employed or combined with these approaches.
Contraindications
Absolute Contraindications:
- Coagulopathies and bleeding disorders present a heightened risk during surgical implantation.
- Immunosuppression in individuals, particularly those with cancer, faces increased risks of postprocedural infections.
- Infection at the implantation site poses a direct risk of introducing infection into the intrathecal space.
- Systemic infections pose a risk of spreading through the device into the CSF.
- Known allergies to implanted materials or administered medications are contraindications.
- Active intravenous drug use increases the risk of infection and complicates postoperative management.
- Psychosis or dementia can affect patient compliance and the ability to manage the pump.
- Patients with a life expectancy of 3 months or less may benefit more from less invasive options, such as external infusion systems.
- Patient refusal is a contraindication.[11]
Relative Contraindications:
- Smoking and poor glycemic control have been shown to increase the risk of surgical site infections. Ideally, cessation of smoking for 4 weeks and an A1C of 7% or less should be targeted before surgery.
- Epidural metastases and spinal stenosis may limit drug distribution to the targeted nerve roots and spinal cord, affecting efficacy.
- Low body mass index presents challenges in safely implanting and managing the pump device.
- Active anticoagulation therapy increases the risk of bleeding and should be temporarily suspended if it is deemed safe to do so.
- Active bleeding poses immediate risks during and after the surgical procedure.
- High opioid tolerance may limit the effectiveness of intrathecal opioid therapy.
- Lack of social or family support may compromise postoperative care and pump management.
- Lack of access to medical care may negatively affect ongoing maintenance and management.
- Cardiovascular and cerebrovascular diseases may exclude a patient from undergoing this procedure due to increased risks.
The Recommendations on Intrathecal Drug Infusion Systems Best Practices and Guidelines from the 2017 Polyanalgesic Consensus Conference (PACC) advise against intrathecal analgesia for widespread pain, headaches, or facial pain due to poor efficacy.[12] Intrathecal pump placement is an elective procedure; this underscores the importance of thoroughly assessing all potential contraindications before therapy. A comprehensive evaluation tailors a multimodal approach to individual needs and conditions.[13]
Equipment
The equipment typically required for placement of an implantable intrathecal drug delivery system includes:
- Fluoroscopy
- Touhey needle
- An implantable intrathecal infusion pump or device
- Intrathecal catheter
- Appropriate surgical instruments for implantation
- Local anesthetics for infiltration
- Drug to be placed in the intrathecal infusion pump
- External programmer for the pump.
Drugs
Three drugs are currently FDA-approved for intrathecal administration: morphine, ziconotide, and baclofen. However, other opioid medications, such as hydromorphone, fentanyl, and sufentanil, and the nonopioids bupivacaine and clonidine, used alone or in combination, are recommended by the PACC for intrathecal administration.[14] Morphine and ziconotide are both recommended for intrathecal monotherapy for treating neuropathic and nociceptive pain. When choosing the drug, clinicians must take into consideration the characteristics of the patient as well as the advantages and disadvantages of each drug.
Morphine with and without bupivacaine
There is a high density of morphine-targeted opioid receptors in the dorsal horn of the spinal cord, where morphine binds to receptors on primary afferent presynaptic neuronal fibers and postsynaptic neurons. The binding of morphine, termed hyperpolarization, inhibits the release of neurotransmitters such as substance P and the calcitonin gene-related peptide, effectively inhibiting nociceptive transmission. Intrathecal morphine has a better analgesic effect and causes fewer adverse events than systemic morphine. Morphine is hydrophilic, remains localized in the intrathecal space, and spreads to different levels of the spinal cord, in contrast to lipophilic opioids such as fentanyl. These pharmacokinetic features offer superior pain control and a significant reduction in drug toxicity. A continuous intrathecal infusion allows the drug to reach and maintain a steady state, resulting in a better therapeutic effect than oral administration.[15]
The opioid dosage and the occurrence of adverse events are directly related; morphine dosages must be kept as low as possible. The most appropriate intrathecal morphine dosage is based on the test dose; test dosing is rarely performed for cancer-related pain. However, the recommended dosage corresponds to 1/300th of the patient's total oral morphine dosage; excellent effects may be achievable with much lower doses.[16][17] Maintaining analgesia may require increased dosing as tolerance develops.
Morphine-induced adverse events include respiratory depression, pruritus from histamine release, nausea and vomiting due to stimulation of the chemoreceptor trigger zone in the fourth ventricle, and sedation due to effects on the thalamus and limbic system. Compared to the parenteral route, the intrathecal administration of morphine can lead to an increased occurrence of urinary retention due to inhibition of the sacral parasympathetic system and relaxation of the detrusor muscle. In some cases, urinary catheterization is necessary; urinary retention often resolves with the cessation of the infusion and naloxone administration.
The addition of local anesthetics such as bupivacaine to the intrathecal morphine preparation can enhance analgesic effects while reducing the required dosage. Adverse events commonly associated with bupivacaine, such as hypotension and muscle weakness, are rarely observed. Therefore, using bupivacaine and morphine in concert can achieve effective pain management with few side effects. The exact mechanism of action behind their synergistic effect is not fully understood but may be due to changes in the sodium and potassium electrochemical gradients, which affect neurotransmitter release in the spinal cord and improve the cholinergic transmission of the pain signaling system. The effect of the local anesthetic infusion alone as a treatment for cancer pain requires additional investigation. Results from a recent randomized, double-blind, cross-over trial demonstrated that in cancer pain, the continuous intrathecal administration of the bupivacaine alone could lead to better analgesia than the association of the local anesthetic with morphine.[18]
Ziconotide
Ziconotide is a nonopioid analgesic derived from the ω-conotoxin of the marine gastropod Conus magus. ω-conotoxin selectively binds to N-type voltage-sensitive calcium channels (Cav2.2) on nociceptive afferent nerves in the spinal cord dorsal horn, blocking the release of pronociceptive neurotransmitters and causing analgesia. Ziconotide was FDA-approved in 2004 for analgesic use via intrathecal administration. In 2012, the PACC guidelines recommended ziconotide as a first-line intrathecal therapy for neuropathic and nociceptive pain.[19] In 2017, the PACC guidelines emphasized the importance of intrathecal treatment not just as a last resort following high-dose systemic therapy failure but as a viable primary analgesic strategy without distinguishing between pain types.[20]
Intrathecally administered ziconotide does not exhibit any cardiopulmonary adverse events or physical dependence and lacks withdrawal symptoms upon therapy interruption. However, ziconotide is not without drawbacks. Common adverse events experienced by more than 25% of patients include dizziness, nausea, confusion, and nystagmus. Less than 5% of patients treated with ziconotide experience urinary retention, blurred vision, double vision, diarrhea, vomiting, constipation, limb pain, muscle spasms, ataxia, drowsiness, tremor, and altered taste. Systemic symptoms such as fever, weakness, and loss of appetite are reported by less than 5% of patients who used intrathecal ziconotide. Psychological effects such as anxiety, insomnia, depression, and, in rare instances, psychosis with hallucinations and paranoia, have also been reported. Because of the possibility of these psychiatric manifestations, ziconotide is contraindicated in patients with preexisting histories of psychosis. Moreover, particular caution should be exercised when considering starting ziconotide in patients with a psychiatric history or those who are predisposed to suicidal ideation. Halving the ziconotide dosage when adverse effects become problematic is recommended.[14][21]
Ziconotide has a narrow therapeutic window; therefore, the dosage titration must be accurate. The initial dosage of continuously administered intrathecal ziconotide should be less than or equal to 1.2 mcg/d. To minimize adverse events, the dosage should be slowly titrated upward by increments of less than or equal to 0.5 mcg/d every 4 to 7 days; the recommended maximum dosage is 19.2 mcg/d.[22][23] Ziconotide does not induce tolerance; dose escalation is generally unnecessary, and doses may be reducible over time.
Baclofen
Baclofen is a muscle relaxant and antispasmodic agent initially introduced in 1962 as an anticonvulsant and later repurposed due to adverse effects. As a gamma-aminobutyric acid (GABA) derivative, baclofen activates GABA-B receptors in the spinal cord and brain, suppressing stretch reflexes and reducing muscle tone. Oral administration of baclofen presents challenges; only a small portion of the dose effectively crosses the blood-brain barrier to impact central nervous system receptors. Approximately 25% to 30% of patients with spasticity from spinal cord injuries and multiple sclerosis do not respond to baclofen therapy because of limited central nervous system distribution.[24] Delivering baclofen directly into the intrathecal space began in 1984 and offers increased efficacy with fewer adverse effects than oral administration.
Initiating intrathecal baclofen therapy requires titration. The recommended titration regimen begins by administering a single test dose, such as 50 mcg, and monitoring the patient for 5 to 10 hours to evaluate effectiveness. The dose is then adjusted (eg, usually increased by 10% to 30% for spinal cord-origin spasticity and 5% to 15% for cerebral-origin spasticity) until the optimal daily dosage is determined.
The main adverse events of baclofen include sedation, excessive muscle weakness, nausea, dizziness, mental confusion, and drowsiness; these adverse events are generally less pronounced with intrathecal administration.[25] Severe toxicity can result in flaccid paralysis, respiratory failure, cardiac arrhythmias, or hemodynamic collapse. The adverse events of baclofen tend to be dose-dependent. Despite up to 27% of patients discontinuing treatment due to these events, starting low and gradually increasing the dosage can mitigate these risks. Results from recent studies, including a retrospective analysis of using intrathecal baclofen for treating spasticity in children, have demonstrated the efficacy and feasibility of this method, even in children younger than 6 years.[26]
Non-FDA-approved intrathecal drugs
Clonidine, an alpha-2 agonist, treats pain in a dose-dependent manner by inhibiting neuropathic pain pathways and releasing pro-inflammatory cytokines. The recommendation is to use clonidine in combination with at least 2 other classes of intrathecal analgesics. Side effects include hypotension, bradycardia, sedation, insomnia, nausea, confusion, and xerostomia. Interestingly, the cardiovascular events seen with intrathecal clonidine are inversely proportional to the dosing (ie, more pronounced at lower doses). Additionally, clinicians must be wary of rebound hypertension that could result from a sudden cessation of the drug.
Local anesthetics are another type of drug that can be delivered intrathecally. These act by blocking voltage-gated sodium channels in neurons, thus inhibiting the propagation of action potentials. Bupivacaine is the only local anesthetic recommended per PACC guidelines; this drug can be used alone in the treatment of localized non-cancer pain or as an adjunct to an opioid in cases of inadequate analgesia.
Implantable Intrathecal Drug Delivery Device
The implantable intrathecal drug delivery device comprises 3 components: the programmable pump that contains and infuses the drug, the catheter that serves as a conduit from the pump to the intrathecal space, and the external device, or programmer, that permits supplemental drug doses when needed. The pump is an adjustable-flow mechanical pump powered by a lithium battery with an average life of 5 to 7 years. The pump has an antenna that allows communication with the programmer via computer. The pump contains a 20- to 40-mL refillable drug reservoir; refilling is performed via a thin needle introduced through the skin. The reservoir should be refilled approximately every 6 months, but this interval varies with the type of disease being treated, the drug, and its dosage.
The adjustable-flow pump is equipped with several infusion modes. The continuous (fixed) infusion mode administers the drug continuously 24 hours a day. The flexible infusion mode permits the clinician to change the daily dosage of the drug, increase or decrease the drug at any time during the day, deliver variable quantities at different times, and customize the therapy based on the patient's needs. The patient-controlled analgesia infusion mode allows continuous administration and bolus on-demand doses when the patient needs it, as in cases of incident pain such as breakthrough cancer pain.
Personnel
Intrathecal drug delivery system implantation is performed primarily by neurosurgeons, interventional pain management physicians, and orthopedic spine specialists. Other personnel typically involved in the procedure include:
- Fluoroscopy technician
- Surgical technician or operating room nurse
- Circulating or operating room nurse
- Anesthesia personnel
Preparation
A thorough medical history should be obtained, and a comprehensive physical examination should be performed. A detailed discussion of the risks and benefits of the procedure should be reviewed to facilitate shared decision-making. Imaging is essential when assessing spinal anatomy and determining the location of needle and catheter placement; preprocedural magnetic resonance imaging (MRI) of the spine is preferred. The optimal needle and catheter entry point is typically below the level of the conus medullaris between the L2 and S1 lumbar interspaces. Therefore, an MRI of the lumbar spine is required; a thoracic and cervical MRI may be necessary depending on the preferred ultimate location of the catheter tip.
Laboratory studies should be performed in the immediate preoperative period. These studies should include a complete blood count with platelets, prothrombin time, and partial thromboplastin time; platelet function studies or a bleeding time may be required. Patients taking anticoagulants require consultation with the prescribing physician regarding the feasibility of temporarily suspending anticoagulation therapy in the immediate perioperative period.
When treating spasticity, administering a test dose of the chosen medication a few days to weeks before the procedure is standard. Adminstration of a test dose for patients with chronic pain is discretionary; a test dose is rarely given for cancer-related pain. The test dose can be administered continuously through a catheter connected to an external pump. Test positivity is indicated by pain reduction of at least 50% compared to baseline. The single bolus may be repeated every 24 hours with increasing doses of the medication scheduled to be delivered through the intrathecal drug delivery system (IDDS). The lack of a clinical response must lead to a reevaluation of the patient. The effective test dose represents the target dose to be delivered through the IDDS. However, to limit postimplant drug-induced adverse events, the initial daily dose can be achieved by reducing the effective test dose to which the patient responded positively by 20% and increasing the dose by 20% after 3 to 5 days to reach the target dose.
The implantation procedure is performed in the operating room with local anesthesia and conscious sedation or general anesthesia. The advantages of conscious sedation include a potentially lower risk of spinal cord or nerve root damage as the patient can undergo real-time assessment of their neurologic status. However, many clinicians prefer general anesthesia to reduce pain, mitigate patient anxiety, and limit patient movement that may lead to complications.[27] Antibiotic prophylaxis should be given within 1 hour of skin incision. The current recommendation is weight-based intravenous cefazolin; vancomycin is administered if the patient has a penicillin or cephalosporin allergy.
Technique or Treatment
The clinician and the patient should share preoperative decision-making regarding the subcutaneous anatomical site for pump placement. The current on-label locations for implantation are the right or left lower abdominal quadrants. The anatomical boundaries are the iliac crest and costal margin; these areas should not make contact with the implanted pump.
Procedural Technique
An intrathecal drug delivery system is implanted with the patient in the lateral decubitus position; the side chosen for pump implantation should be upward. Sterilely prepare the back and abdomen. Employ C-arm fluoroscopy to confirm access to the intrathecal space and identify the location of the catheter tip. Position the C-arm for the appropriate anterior-posterior view, allowing easy lumbar puncture, visualization of catheter threading, and tip position.
Incise the skin and carry the incision down to the dorsolumbar fascia to expose the supraspinous ligament. Place a Tuohy needle into the intrathecal space through the exposed tissue using a paramedian approach (see Image. Fluoroscopy, Intrathecal Catheter Placement). To reduce spinal cord injury, the intrathecal space should be entered below the level where the spinal cord ends (L1 or L2). A slight cephalad needle angle optimizes CSF flow and decreases the risk of catheter kink or fracture. After confirming adequate CSF flow, thread the catheter through the Tuohy needle, confirming the catheter and catheter tip position with intermittent fluoroscopy. Place 2 fascial sutures on either side of the Tuohy needle while keeping the Tuohy needle in place to prevent accidental catheter damage. Once the final catheter tip positioning is confirmed, remove the introducer Tuohy needle, withdraw the needle and guidewire together, leaving the catheter in place, and recheck for CSF back-flow. Insert the catheter into an anchor and secure it with fascial sutures. After securing the catheter to the dorsolumbar fascia, clamp the distal intrathecal catheter to prevent CSF loss.
Turn attention to the chosen abdominal lower quadrant. Make an approximately 8 cm lengthwise incision down to the underlying subcutaneous fat layer. Create a subcutaneous pocket large enough to contain the pump being used. Generally, if all 4 fingers can fit into the pocket, the pocket is large enough to allow closure without tension. The depth of the pocket below the skin is critical for programmable pumps. A depth greater than 2.5 cm may not reliably permit telemetry, making pump refilling more difficult. Ensure hemostasis at both incision sites to avoid postoperative hematoma formation. Irrigate the pockets with antibiotic solution.
Assemble the malleable tunneling device and prepare a subcutaneous tunnel from the midline back incision to the pump pocket. Once the tunneling device is successfully placed, push the catheter through the tunnel to the abdominal pocket. Cut any excess catheter but ensure adequate length for a stress relief loop in the midline incision and pump pocket. Catheters that are too long are susceptible to kinking. Attach the catheter to the pump nipple and verify it is secure.
Place the programmable pump into the subcutaneous abdominal pocket. Stitches may be placed through the pump's anchoring loops to prevent rotation. If this technique is used, the stitches should be placed into the pocket first, then through the pump suture loops. Carefully close the pocket and spinal incisions with suture; approximate the skin without tension.
Complications
Procedure-Related Complications
Bleeding
Abdominal incision: Careful attention to intraoperative hemostasis can avoid the formation of a hematoma within the abdominal pocket. Using an abdominal binder in the early postoperative period may also reduce the formation of hematoma or seroma in the abdominal wound.
Epidural or intrathecal bleed: Preoperative anticoagulation reversal and cessation of corticosteroid or anti-inflammatory medications will reduce the risk of this complication. Signs of a developing epidural or intrathecal hematoma include an abrupt increase in back pain, progressive numbness, weakness in the lower extremities, and loss of bowel and bladder control. This clinical presentation justifies immediate myelography with MRI or computed tomography (CT); neurologic deterioration warrants emergent surgical decompression.
Infection
Wound infection: Prophylactic antibiotics and intraoperative antibiotic wound irrigation are usually sufficient to prevent wound infection when combined with appropriate adherence to sterile technique. Superficial wound infection may be managed conservatively. Deeper wound infections require device explantation; infection can track along the intrathecal catheter and can cause either meningitis or an epidural abscess.[28]
Injury to surrounding tissue
Nerve root injury: Needle placement, even when performed with fluoroscopic guidance, may injure the nerve roots. However, placing the catheter under conscious sedation can decrease this risk. Patients under conscious sedation can report a shock-like or burning sensation in the distribution of the involved nerve root; if this occurs, the needle should be promptly withdrawn and placed at a different level.
Spinal cord injury: Catheters are designed with some stiffness to facilitate steering through the intrathecal space. The catheter must not be forced through the spinal canal because the tip may end up in an intramedullary position. Penetration of the spinal cord often results in dysesthesias or burning, stinging pain below the lesion, and neurological signs are usually observable immediately. Intramedullary infusion of the drug may result in developing signs of a spinal cord lesion and should be immediately evaluated with MRI or CT myelography and followed by a neurosurgical consultation.
Cerebral Spinal Fluid Leak
CSF leaks can occur either due to failure of the dura to close over time after insertion of the catheter or several causes of catheter injury. CSF leakage can evolve into a postdural puncture headache. The initial management of a postdural puncture headache is conservative, comprising intravenous fluid administration, supine positioning, increased caffeine intake, and utilization of nonopioid analgesics such as acetaminophen. If the headache is refractory to conservative management, a blood patch of 10 to 20 mL of autologous venous blood injected one level above the catheter entry point under fluoroscopic guidance should treat the headache effectively.[29]
Sometimes, the CSF may leak into the pump pocket, creating a noticeable swelling in the abdomen. To confirm the origin of the fluid, it should be aspirated and sent for detection of beta-2-transferrin. A serious sequela of CSF leak is intracranial hypovolemia; this should be considered a medical emergency, as it can result in subdural hematoma. The treatment of CSF leaks necessitates the identification of the underlying cause. As described above, blood patches can be performed if the culprit is an opening in the dura. In cases of device-related issues, surgical intervention may be required.
Device-Related Complications
Device-related complications typically involve either the catheter system or the pump. Catheter-related complications are more frequent than pump complications.[30]
Catheter malfunction
Catheter malfunction is frequently suspected when the expected and measured residual infusion volumes differ by more than 20%, or the patient reports a fluctuation in analgesic effectiveness. A comprehensive evaluation of the catheter is necessary if kinking, obstruction, or separation is suspected. This evaluation is performed with basic imaging or a catheter study; catheter studies require injecting contrast dye via the pump side port. Injecting contrast can display the point of kinking, obstruction, or leakage. The catheter should be aspirated before injecting contrast dye to avoid delivering a large dose of medication into the intrathecal space, which can lead to overdosage.
Intrathecal granuloma formation
In addition to catheter tip obstruction, intrathecal granulomas may increase pain and neurologic deficits. The risk of granuloma progression seems to correlate directly with the daily opioid dose, the rate of drug titration, and the duration of intrathecal therapy. This is most commonly seen when using morphine, which is postulated to induce a cascade reaction involving mast cells that leads to increased cytokine formation and inflammatory response. If a granuloma is suspected, the diagnosis should be confirmed with an MRI, preferably with contrast.[31] Reducing the medication dose, switching to another medication, or relocating the catheter will typically lead to the resolution of the granuloma. In patients with progressive neurologic deficits, however, surgical intervention should be considered.
Clinical Significance
The safety and efficacy of intrathecal drug delivery for controlling spasticity and cancer-related and noncancer pain have been widely demonstrated. An implantable drug delivery system provides the advantage of using comparably low daily dosages to achieve the desired analgesic effect and diminished systemic adverse events relative to oral or intravenous administration of the same agent. The implantable device regulates the daily infusion rate to eliminate the peaks and troughs in plasma drug levels seen with oral dosing.[32]
However, intrathecal treatment is not without risk; risk reduction is facilitated by management within specific centers by a multidisciplinary team that can adequately select the patient by evaluating the risks and benefits of an invasive therapy and the complications and costs to obtain the best results. A team that collaborates with an oncologist, radiologist, surgeon, anesthesiologist, palliative care specialist, pain medicine specialist, and nurses is desirable. The selection of the patient, the timing of catheter implantation, and the rapid identification of complications and adverse effects are critical to therapeutic success.
Enhancing Healthcare Team Outcomes
Recognizing that an integrated healthcare team approach should be utilized to manage chronic pain in which intrathecal drug delivery is indicated is vital. Intrathecal therapy and management can substantially lower healthcare costs compared with conventional pain management, especially when systemic opioids are discontinued.[33] To attain beneficial patient-centric care, the supervising clinician should oversee pump refills and medication adjustments after patient evaluation.
The PACC guidelines contain algorithms intended to guide intrathecal medication choices for localized or diffuse nociceptive or neuropathic pain for patients with cancer, terminal illness, and noncancer pain. These guidelines emphasize the necessity for clinical education across healthcare settings and specialties to address the expanding evidence related to intrathecal drug delivery for chronic pain. Protocols for patients with an implantable intrathecal drug delivery system should be assembled around a collaborative team-based care model that provides ongoing education for patients and caregivers and properly uses psychosocial support services. A patient-centered approach can lend to encouraging outcomes among patients with refractory chronic pain.[34] All nurses caring for patients with an intrathecal pump should have an orientation and a seminar to ensure they are fully aware of the potential therapeutic complications.
Media
(Click Image to Enlarge)
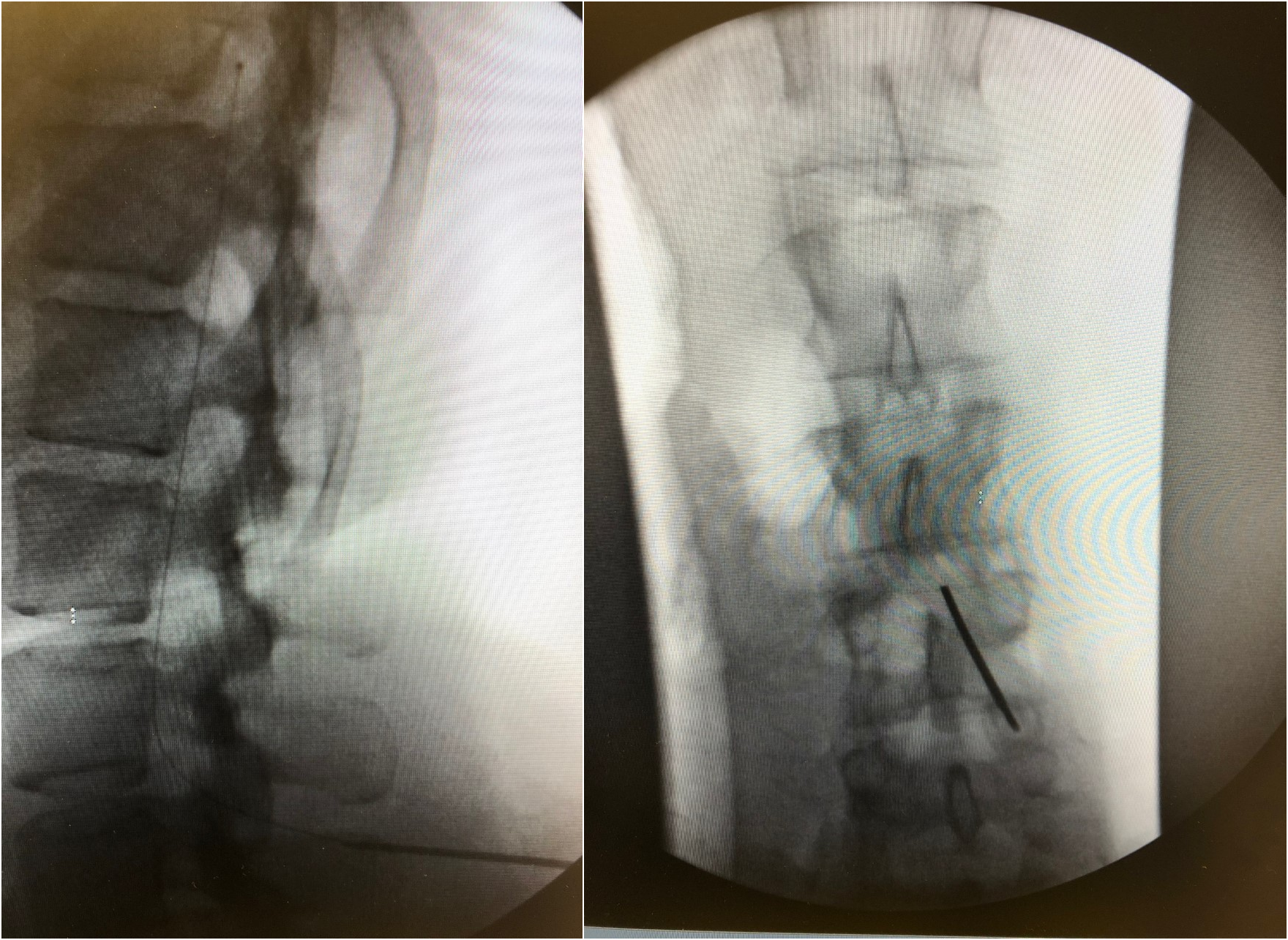
Fluoroscopy, Intrathecal Catheter Placement. The image on the left is a lateral fluoroscopic view demonstrating a catheter in the intrathecal space. The image on the right demonstrates the characteristic paramedian Tuohy needle approach to obtain intrathecal access for catheter placement.
Contributed by D Padalia, MD
References
Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychological bulletin. 2007 Jul:133(4):581-624 [PubMed PMID: 17592957]
Level 3 (low-level) evidenceWhitten CE, Donovan M, Cristobal K. Treating chronic pain: new knowledge, more choices. The Permanente journal. 2005 Fall:9(4):9-18 [PubMed PMID: 22811639]
Jay GW, Barkin RL. Perspectives on the opioid crisis from pain medicine clinicians. Disease-a-month : DM. 2018 Oct:64(10):451-466. doi: 10.1016/j.disamonth.2018.07.002. Epub 2018 Sep 18 [PubMed PMID: 30236900]
Level 3 (low-level) evidenceSimpson KH, Jones I. Intrathecal drug delivery for management of cancer and noncancer pain. Journal of opioid management. 2008 Sep-Oct:4(5):293-304 [PubMed PMID: 19070267]
Prager J, Deer T, Levy R, Bruel B, Buchser E, Caraway D, Cousins M, Jacobs M, McGlothlen G, Rauck R, Staats P, Stearns L. Best practices for intrathecal drug delivery for pain. Neuromodulation : journal of the International Neuromodulation Society. 2014 Jun:17(4):354-72; discussion 372. doi: 10.1111/ner.12146. Epub 2014 Jan 21 [PubMed PMID: 24446870]
Kroin JS. Intrathecal drug administration. Present use and future trends. Clinical pharmacokinetics. 1992 May:22(5):319-26 [PubMed PMID: 1380410]
Schuster M, Bayer O, Heid F, Laufenberg-Feldmann R. Opioid Rotation in Cancer Pain Treatment. Deutsches Arzteblatt international. 2018 Mar 2:115(9):135-142. doi: 10.3238/arztebl.2018.0135. Epub [PubMed PMID: 29563006]
van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ. Update on Prevalence of Pain in Patients With Cancer: Systematic Review and Meta-Analysis. Journal of pain and symptom management. 2016 Jun:51(6):1070-1090.e9. doi: 10.1016/j.jpainsymman.2015.12.340. Epub 2016 Apr 23 [PubMed PMID: 27112310]
Level 1 (high-level) evidence. WHO Guidelines for the Pharmacological and Radiotherapeutic Management of Cancer Pain in Adults and Adolescents. 2018:(): [PubMed PMID: 30776210]
Cuomo A, Bimonte S, Forte CA, Botti G, Cascella M. Multimodal approaches and tailored therapies for pain management: the trolley analgesic model. Journal of pain research. 2019:12():711-714. doi: 10.2147/JPR.S178910. Epub 2019 Feb 19 [PubMed PMID: 30863143]
Hassenbusch SJ, Paice JA, Patt RB, Bedder MD, Bell GK. Clinical realities and economic considerations: economics of intrathecal therapy. Journal of pain and symptom management. 1997 Sep:14(3 Suppl):S36-48 [PubMed PMID: 9291709]
. The Polyanalgesic Consensus Conference (PACC): Recommendations on Intrathecal Drug Infusion Systems Best Practices and Guidelines. Neuromodulation : journal of the International Neuromodulation Society. 2017 Jun:20(4):405-406. doi: 10.1111/ner.12618. Epub [PubMed PMID: 28593641]
Level 3 (low-level) evidenceDeer TR, Smith HS, Cousins M, Doleys DM, Levy RM, Rathmell JP, Staats PS, Wallace M, Webster LR. Consensus guidelines for the selection and implantation of patients with noncancer pain for intrathecal drug delivery. Pain physician. 2010 May-Jun:13(3):E175-213 [PubMed PMID: 20495597]
Level 3 (low-level) evidenceDeer TR, Pope JE, Hanes MC, McDowell GC. Intrathecal Therapy for Chronic Pain: A Review of Morphine and Ziconotide as Firstline Options. Pain medicine (Malden, Mass.). 2019 Apr 1:20(4):784-798. doi: 10.1093/pm/pny132. Epub [PubMed PMID: 30137539]
Dupoiron D. Intrathecal therapy for pain in cancer patients. Current opinion in supportive and palliative care. 2019 Jun:13(2):75-80. doi: 10.1097/SPC.0000000000000427. Epub [PubMed PMID: 30896454]
Level 3 (low-level) evidenceBhatia G, Lau ME, Koury KM, Gulur P. Intrathecal Drug Delivery (ITDD) systems for cancer pain. F1000Research. 2013:2():96. doi: 10.12688/f1000research.2-96.v4. Epub 2013 Mar 28 [PubMed PMID: 24555051]
Qin W, Liu B, Deng A, Liu Y, Zhang X, Zhang L. Super analgesia of intrathecal morphine may be related to ABCB1 (MDR1) gene polymorphism. Journal of pain research. 2018:11():1355-1357. doi: 10.2147/JPR.S156919. Epub 2018 Jul 20 [PubMed PMID: 30050319]
Level 2 (mid-level) evidenceReif I, Wincent A, Stiller CO. Intrathecal analgesia by bupivacaine is not enhanced by coadministration of morphine in patients with severe cancer-related pain: a randomized double-blind cross-over study. International journal of clinical pharmacology and therapeutics. 2017 Jun:55(6):525-532. doi: 10.5414/CP202955. Epub [PubMed PMID: 28291511]
Level 1 (high-level) evidenceDeer TR, Prager J, Levy R, Rathmell J, Buchser E, Burton A, Caraway D, Cousins M, De Andrés J, Diwan S, Erdek M, Grigsby E, Huntoon M, Jacobs MS, Kim P, Kumar K, Leong M, Liem L, McDowell GC 2nd, Panchal S, Rauck R, Saulino M, Sitzman BT, Staats P, Stanton-Hicks M, Stearns L, Wallace M, Willis KD, Witt W, Yaksh T, Mekhail N. Polyanalgesic Consensus Conference 2012: recommendations for the management of pain by intrathecal (intraspinal) drug delivery: report of an interdisciplinary expert panel. Neuromodulation : journal of the International Neuromodulation Society. 2012 Sep-Oct:15(5):436-64; discussion 464-6. doi: 10.1111/j.1525-1403.2012.00476.x. Epub 2012 Jul 2 [PubMed PMID: 22748024]
Level 3 (low-level) evidenceDeer TR, Pope JE, Hayek SM, Bux A, Buchser E, Eldabe S, De Andrés JA, Erdek M, Patin D, Grider JS, Doleys DM, Jacobs MS, Yaksh TL, Poree L, Wallace MS, Prager J, Rauck R, DeLeon O, Diwan S, Falowski SM, Gazelka HM, Kim P, Leong M, Levy RM, McDowell G II, McRoberts P, Naidu R, Narouze S, Perruchoud C, Rosen SM, Rosenberg WS, Saulino M, Staats P, Stearns LJ, Willis D, Krames E, Huntoon M, Mekhail N. The Polyanalgesic Consensus Conference (PACC): Recommendations on Intrathecal Drug Infusion Systems Best Practices and Guidelines. Neuromodulation : journal of the International Neuromodulation Society. 2017 Feb:20(2):96-132. doi: 10.1111/ner.12538. Epub 2017 Jan 2 [PubMed PMID: 28042904]
Level 3 (low-level) evidenceMcGivern JG. Ziconotide: a review of its pharmacology and use in the treatment of pain. Neuropsychiatric disease and treatment. 2007 Feb:3(1):69-85 [PubMed PMID: 19300539]
Pope JE, Deer TR, Bruel BM, Falowski S. Clinical Uses of Intrathecal Therapy and Its Placement in the Pain Care Algorithm. Pain practice : the official journal of World Institute of Pain. 2016 Nov:16(8):1092-1106. doi: 10.1111/papr.12438. Epub 2016 Feb 23 [PubMed PMID: 26914961]
McDowell GC 2nd, Pope JE. Intrathecal Ziconotide: Dosing and Administration Strategies in Patients With Refractory Chronic Pain. Neuromodulation : journal of the International Neuromodulation Society. 2016 Jul:19(5):522-32. doi: 10.1111/ner.12392. Epub 2016 Feb 9 [PubMed PMID: 26856969]
Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A, Parodi L, Rydning SL, Tallaksen C, Durr A. Spastic Paraplegia 4. GeneReviews(®). 1993:(): [PubMed PMID: 20301339]
Abe T, Taniguchi W, Nishio N, Nakatsuka T, Yoshida M, Yamada H. Molecular mechanisms of the antispasticity effects of baclofen on spinal ventral horn neurons. Neuroreport. 2019 Jan 2:30(1):19-25. doi: 10.1097/WNR.0000000000001155. Epub [PubMed PMID: 30371538]
Hagemann C, Schmitt I, Lischetzki G, Kunkel P. Intrathecal baclofen therapy for treatment of spasticity in infants and small children under 6 years of age. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2020 Apr:36(4):767-773. doi: 10.1007/s00381-019-04341-7. Epub 2019 Aug 9 [PubMed PMID: 31399764]
Parida S, Kundra P, Mohan VK, Mishra SK. Standards of care for procedural sedation: Focus on differing perceptions among societies. Indian journal of anaesthesia. 2018 Jul:62(7):493-496. doi: 10.4103/ija.IJA_201_18. Epub [PubMed PMID: 30078850]
Buxton K. Balancing the benefits of intrathecal baclofen pump surgery with potential postoperative complications. Developmental medicine and child neurology. 2018 Oct:60(10):972-973. doi: 10.1111/dmcn.13756. Epub 2018 Mar 25 [PubMed PMID: 29574822]
Buddeberg BS, Bandschapp O, Girard T. Post-dural puncture headache. Minerva anestesiologica. 2019 May:85(5):543-553. doi: 10.23736/S0375-9393.18.13331-1. Epub 2019 Jan 4 [PubMed PMID: 30621376]
Sindt JE, Brogan SE. Interventional Treatments of Cancer Pain. Anesthesiology clinics. 2016 Jun:34(2):317-39. doi: 10.1016/j.anclin.2016.01.004. Epub [PubMed PMID: 27208713]
Krames ES. Intraspinal opioid therapy for chronic nonmalignant pain: current practice and clinical guidelines. Journal of pain and symptom management. 1996 Jun:11(6):333-52 [PubMed PMID: 8935137]
Deer TR, Pope JE, Hayek SM, Lamer TJ, Veizi IE, Erdek M, Wallace MS, Grider JS, Levy RM, Prager J, Rosen SM, Saulino M, Yaksh TL, De Andrés JA, Abejon Gonzalez D, Vesper J, Schu S, Simpson B, Mekhail N. The Polyanalgesic Consensus Conference (PACC): Recommendations for Intrathecal Drug Delivery: Guidance for Improving Safety and Mitigating Risks. Neuromodulation : journal of the International Neuromodulation Society. 2017 Feb:20(2):155-176. doi: 10.1111/ner.12579. Epub 2017 Jan 2 [PubMed PMID: 28042914]
Level 3 (low-level) evidenceHatheway JA, Caraway D, David G, Gunnarsson C, Hinnenthal J, Ernst AR, Saulino M. Systemic opioid elimination after implantation of an intrathecal drug delivery system significantly reduced health-care expenditures. Neuromodulation : journal of the International Neuromodulation Society. 2015 Apr:18(3):207-13; discussion 213. doi: 10.1111/ner.12278. Epub 2015 Feb 17 [PubMed PMID: 25688677]
Level 2 (mid-level) evidenceDeer TR, Hayek SM, Pope JE, Lamer TJ, Hamza M, Grider JS, Rosen SM, Narouze S, Perruchoud C, Thomson S, Russo M, Grigsby E, Doleys DM, Jacobs MS, Saulino M, Christo P, Kim P, Huntoon EM, Krames E, Mekhail N. The Polyanalgesic Consensus Conference (PACC): Recommendations for Trialing of Intrathecal Drug Delivery Infusion Therapy. Neuromodulation : journal of the International Neuromodulation Society. 2017 Feb:20(2):133-154. doi: 10.1111/ner.12543. Epub 2017 Jan 2 [PubMed PMID: 28042906]
Level 3 (low-level) evidence