Introduction
Goodpasture syndrome refers to an anti-glomerular basement membrane (anti-GBM) disease that involves both the lungs and kidneys, often presenting as pulmonary hemorrhage and glomerulonephritis. Belonging to 1 of the 3 main causes of crescentic glomerulonephritis, Goodpasture syndrome often has the worst prognosis, especially when not promptly treated. Please see our companion review, "Rapidly Progressive Glomerulonephritis," for further information and classification of Goodpasture syndrome in the context of the other rapidly progressive glomerulonephritides.
Some clinicians may interchangeably use several terms, including anti-glomerular basement membrane disease, Goodpasture syndrome, and Goodpasture disease.[1] Anti-GBM disease can also refer to the presence of renal disease without pulmonary involvement, while Goodpasture disease or syndrome always has pulmonary involvement. Some clinicians even describe coexistent renal and pulmonary disease of any kind as "Goodpasture Syndrome," but in general, this term refers to anti-GBM antibodies. About 40% to 60% of cases are associated with alveolar hemorrhage, while fewer than 10% of anti-GBM disease cases present with isolated pulmonary involvement.[2][3]
Goodpasture syndrome is an eponym named after Ernest Goodpasture, who first described this disorder in 1919. Immunofluorescence techniques developed in the 1960's and the discovery of anti-GBM antibodies further led to a better understanding of the pathogenesis of Goodpasture syndrome.[4]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Goodpasture syndrome appears to result from environmental or infectious triggers interacting with a genetic predisposition. Currently, a single triggering stimulus has not been identified for developing anti-glomerular basement membrane antibodies, although geographic and seasonal clustering has been noted.
Strong evidence for genetic involvement exists and patients with certain human leukocyte antigen (HLA) types are found to be more susceptible to disease and exhibit a poor prognosis. Most notably, HLA-DR2 has been noted in up to 80% of patients affected by anti-GBM disease. The HLA-DR1 alleles have been noted to confer both negative and positive effects on the development of anti-GBM disease. HLA-DRB1*01 and HLA-DRB1*07 have a protective effect against Goodpasture syndrome, while HLA-DRB1*1501 confers increased susceptibility, particularly in Asian populations.[1][2] These alleles are also found in those with other autoimmune diseases and normal individuals, so HLA testing is not frequently performed.[3][4] For example, the DRB1*1501 allele is present in up to one-third of the White population. Therefore, additional factors are needed for disease expression.
The exposure of the alveolar capillaries to autoantibodies is likely triggered by an initial insult to the pulmonary alveolar or renal glomerular basement membranes. Environmental factors are thought to cause local inflammation and expose basement membrane antigens, which are usually sequestered. Some agents related to disease development include the following:
- Smoking
- Exposure to metal dust, organic solvents, or hydrocarbons
- Bacteremia
- Tobacco smoking
- Endotoxemia
- Exposure to volatile hydrocarbons
- Infections, such as influenza A
- Drugs, such as alemtuzumab, that cause regulatory T-cell depletion
- Inhalation of cocaine
- Extracorporeal shock wave lithotripsy
- Higher inspired oxygen [1][5][6][7]
Epidemiology
Goodpasture syndrome is a rare disorder. The incidence of the anti-GBM disease is approximately 0.5 to 1.8 cases per million per year in Asian and European populations and is responsible for 1% to 5% of all kinds of glomerulonephritides, and it accounts for 10% to 15% of crescentic glomerulonephritis.[8][9][10] Goodpasture syndrome is more common in White patients than Black. However, it may be more prevalent in certain ethnicities, such as the Maori people of New Zealand. The syndrome has a bimodal age distribution around the third and sixth decades of life. Of note, younger patients are more likely to have pulmonary involvement, and older patients are more likely to have less severe renal-limited disease.
Pathophysiology
Goodpasture syndrome is due to circulating autoantibodies directed at the glomerular basement membrane. The resulting crescentic glomerulonephritis is the result of the antigen-antibody complexes that form at the basement membrane.[11] The autoantibodies activate the complement system, causing tissue injury. This binding of autoantibodies can be seen as a linear deposition of immunoglobulins (IgG, and very rarely IgA) along the basement membrane. The inflammatory response in that area results in the typical picture of glomerulonephritis.
Type IV collagen is a main constituent of all basement membranes, which are a specialized form of extracellular matrix, supporting tissue integrity and performing numerous key functions including cell signaling and tissue regeneration.[12] The alveolar basement membrane shares the same collagen target as the glomerulus. Type IV collagen also contains non-collagenous domains. The non-collagenous domain of α-3 (α3NC1) is thought to be the antigen activating the Goodpasture autoantibody. Despite the presence of circulating antibodies, pulmonary symptoms are not always observed. An inciting lung injury seems to make pulmonary symptoms more likely.[13] In a healthy individual, the endothelium acts as a barrier to the anti-basement membrane antibodies. However, if an insult increases the alveolar capillaries' permeability, it leads to the trespassing of autoantibodies, which can then bind to the basement membrane. Although Goodpasture syndrome is regarded as an autoantibody-mediated disease, a vital role is played by T-cells in the initiation and progression of the disease. T-cells cause B-cells to increase antibody production and play a direct role in renal and pulmonary injury.[14]
Double-Positive Antibody Disease
Also called dual antibody disease, this type of crescentic glomerulonephritis is associated with a positive antineutrophil cytoplasmic antibody (ANCA) test and anti-GBM antibody. Some study results have indicated that 10% to 50% of patients with anti-GBM disease have detectable ANCA, typically anti-MPO. In contrast, up to 10% of patients with ANCA-associated vasculitis also have circulating anti-GBM antibodies.[15] Generally, the positive ANCA precedes the anti-GBM antibodies; therefore, it is postulated that ANCA leads to the anti-GBM antibodies by exposing epitopes on the GBM. Patients with double-positive disease are often older, have longer prodromes, and have frequent relapses—similar to ANCA-associated vasculitis. Renal manifestations follow an anti-GBM pattern, whereas systemic symptoms are similar to those of ANCA vasculitis.[16] This double-positive characteristic comes into play during treatment selection.[17][18]
Histopathology
In patients suspected to have Goodpasture syndrome, a renal biopsy should be considered. A kidney biopsy is not only crucial for diagnosis, but the percentage of crescents identified on biopsy confers renal survival prognosis. Lung tissue is typically not sought due to the invasiveness of the procedure and a more difficult immunofixation process for lung tissue.
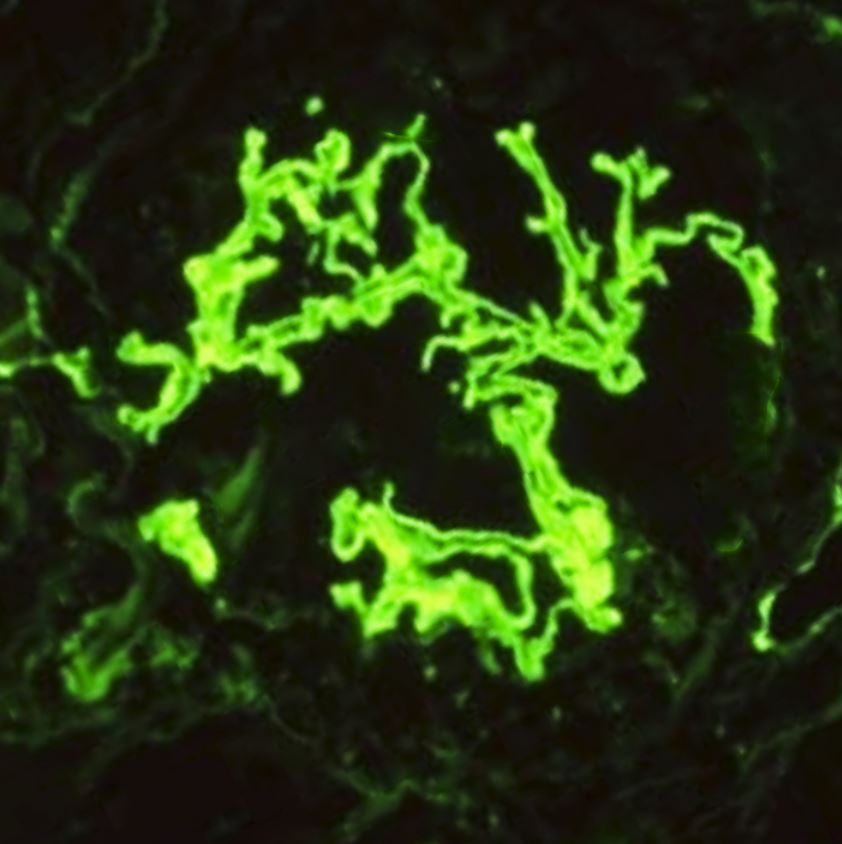
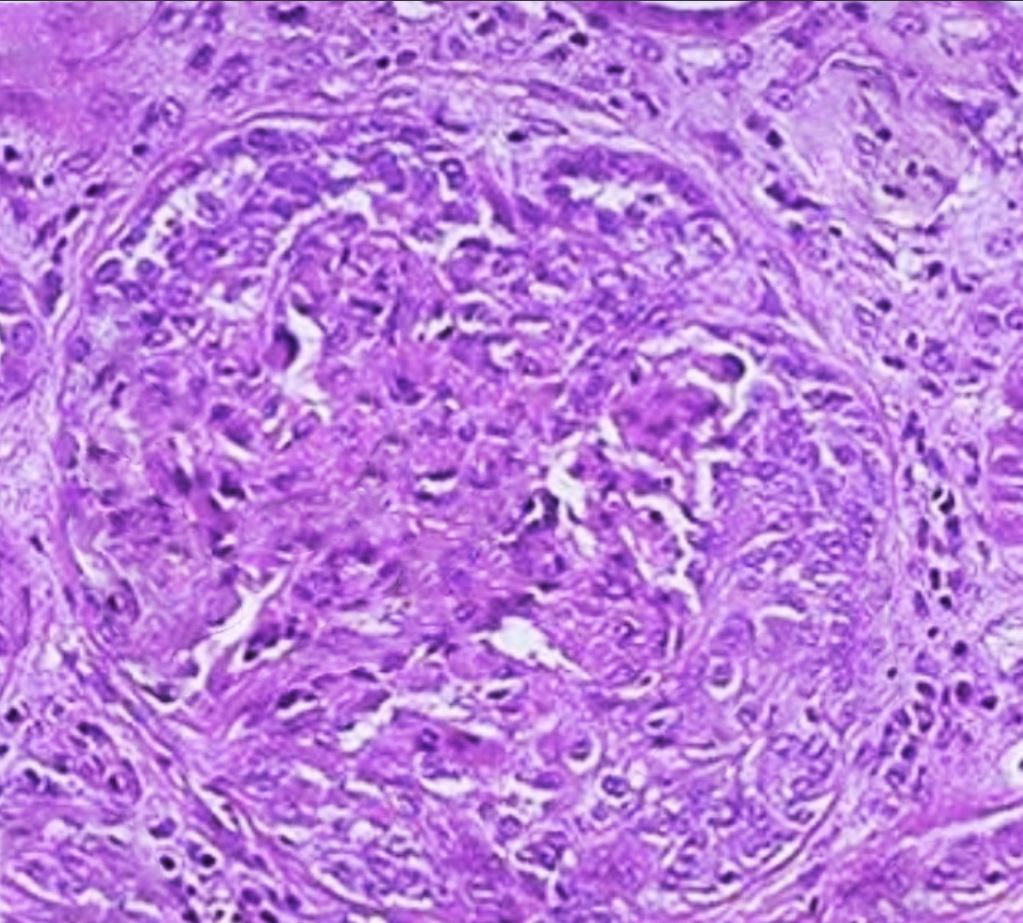
Light microscopy will show crescentic glomerulonephritis. All lesions in anti-GBM disease are generally in the same nephritic stage. Interstitial inflammation is also found mainly in the periglomerular region. In the later stages, fibrosis develops rapidly over a few days to weeks, manifesting as glomerular sclerosis and obliteration. Immunofluorescence stains are more specific and diagnostic, usually exhibiting bright linear deposits of immunoglobulin G (IgG) and complement (C3) on the glomerular basement membrane. Predominantly, the subclass IgG-1 is present.[19] Staining of the renal tubular basement membrane is also possible in addition to glomerular staining.[20] See Images. Crescentic Glomerulonephritis, Immunofluorescence Staining for IgG. Although lung biopsy is not commonly performed, if conducted, similar staining of the alveolar basement membrane can also be observed. The biopsy may also show extensive hemorrhage with deposition of hemosiderin-laden macrophages within alveolar spaces.[1]
A crescent is a hyperplastic lesion involving more than 10% of the glomerulus. If a crescent comprises more than 75% cells and fibrin, with less than 25% being the fibrous matrix, it is called cellular. If the composition comprises 25% to 75% cells/fibrin, with the remainder being the fibrous matrix, the crescent is called fibrocellular. A crescent with less than 25% cells/fibrin and more than 75% fibrous matrix is referred to as a fibrous crescent.[15]
Quantitative definitions aid in the classification of ANCA-associated lesions, and include:
History and Physical
Patients with Goodpasture syndrome will initially present similarly to other forms of rapidly progressive glomerulonephritis with acute renal failure. There are no symptoms specific to the anti-glomerular basement membrane that distinguish it from other diseases causing similar organ dysfunction. The pulmonary symptoms are typically present at the time of the initial encounter or shortly after that. Hemoptysis of varying degrees is typical for pulmonary involvement, ranging from serious and life-threatening bleeding to more subtle diffuse hemorrhage that is only apparent on more careful evaluation.
Younger patients more typically present with simultaneous renal and pulmonary symptoms (Goodpasture syndrome) and tend to be critically ill on presentation. Patients older than 50 tend to present with glomerulonephritis alone and follow a less severe course.
Other physical exam findings to look for in Goodpasture syndrome include the following:
- Increased respiratory rate
- Basilar inspiratory crackles
- Cyanosis
- Hepatosplenomegaly
- Hypertension (in 20% of cases)
- Purpuric rash
- Edema
Evaluation
A kidney biopsy is the gold standard for diagnosis but is not required to begin treatment or continue therapy if a biopsy is not feasible. When performed, a biopsy provides important information regarding the activity and chronicity of renal involvement that may guide therapy. Kidney biopsy is preferable over lung biopsy as it provides a much higher yield, but lung biopsy may be performed where renal biopsy is contraindicated for any reason.
The biopsy tissue should be analyzed using light, electron, and immunofluorescence microscopy. Light microscopy usually shows proliferative or necrotizing crescentic glomerulopathy, as described above. The crescents become fibrotic over time, leading to the development of frank glomerulosclerosis, tubular atrophy, and interstitial fibrosis.[19]
Serologic testing is usually performed by enzyme-linked immunosorbent assay, ELISA, or bead-based fluorescence assays for circulating anti-GBM antibodies. Specifically, the α3NC1 domain area of type IV collagen must be targeted as false positives may be seen in less specific testing.[22] Western blotting may be more specific but is usually available only in research settings. If the etiology is a less common antibody type such as IgG4 or IgA, false negatives are also more likely.[23]
Of note, approximately 10% of patients with biopsy-confirmed anti-GBM disease do not have identifiable circulating antibodies with conventional assays. Therefore, a negative anti-GBM antibody test does not rule out the disease.[23] Chemiluminescence immunoassay is also being studied as a possibly more sensitive test.[1] Quantitative titers are followed during treatment phases to guide treatment decisions.
Other diagnostic testing is as follows:
- Urinalysis usually demonstrates low-grade proteinuria, gross or microscopic hematuria, and red blood cell casts.
- A complete blood count may reveal anemia secondary to intrapulmonary hemorrhages, and leukocytosis is generally present.
- Renal function tests may be deranged secondary to renal dysfunction.
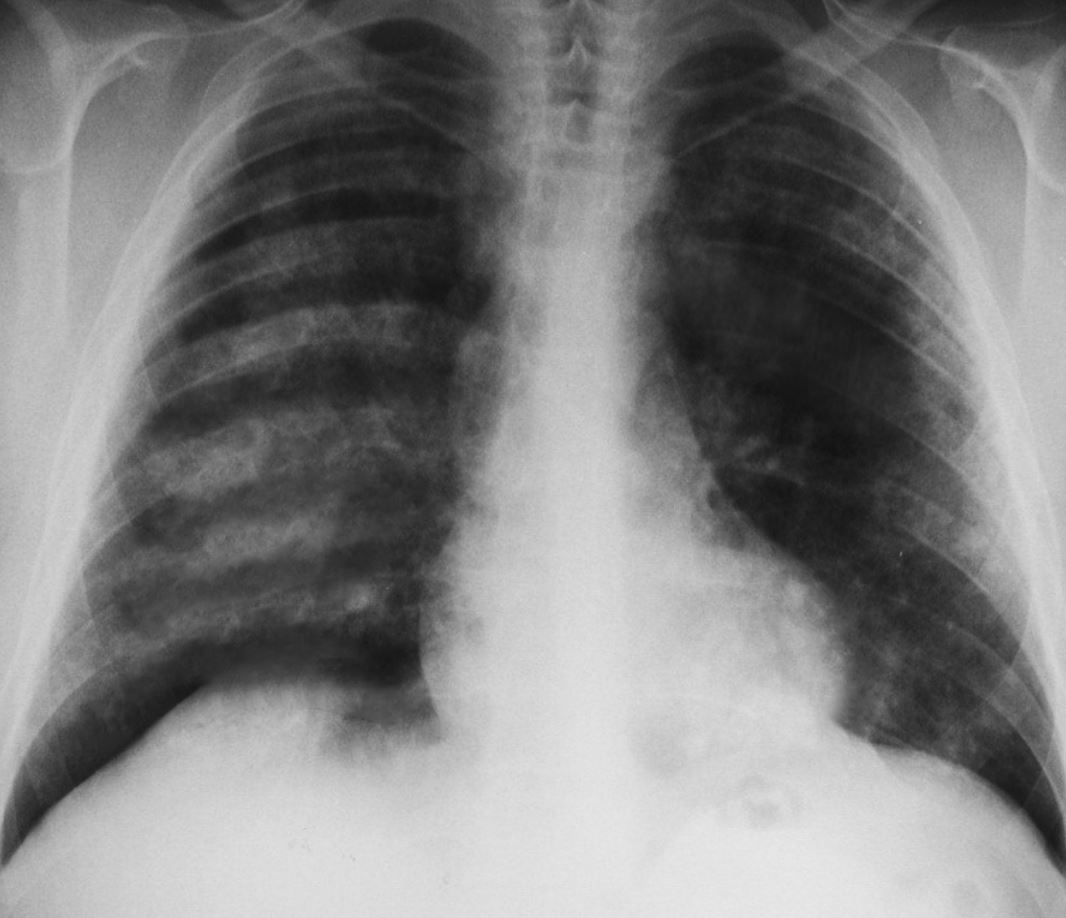
- A chest radiograph shows patchy parenchymal opacifications, which are usually bilateral and bibasilar. The apices and costophrenic angles are generally spared. (see Image. Pulmonary Hemorrhages, Goodpasture Syndrome).
- Pulmonary function tests show an elevated diffusing capacity for carbon monoxide (DLCO) due to its binding to intra-alveolar hemoglobin.
Treatment / Management
Patients presenting with Goodpasture syndrome (both glomerulonephritis and pulmonary hemorrhage) may be critically ill on presentation. Urgent hemodialysis is often needed for standard indications, and intubation for respiratory failure may be necessary. When Goodpasture disease is suspected, a kidney biopsy should be performed as soon as the clinical situation allows. Plasmapheresis, in combination with immunosuppressive agents, is the preferred treatment for anti-GBM disease to remove the autoantibodies. Initiating treatment as early as possible is crucial for preventing progressive renal failure and pulmonary damage.
Recommendations from the 2012 guidelines for Kidney Disease Improving Global Outcomes are initiating immunosuppression with cyclophosphamide plus corticosteroids and plasma exchange in all patients with Goodpasture syndrome, except in cases of patients that are dialysis-dependent at presentation and have 100% crescents on a biopsy without pulmonary hemorrhage. Rituximab can be substituted for cyclophosphamide in cases of adverse affects of cyclophosphamide or concerns for fertility in younger patients.[24]
The dose of plasmapheresis is 4 L of an exchange over 2 to 4 weeks. Typically, 5% albumin is the replacement fluid, but fresh frozen plasma (0.3-2 L) should be administered in cases of invasive procedures or when pulmonary hemorrhage is also noted. Plasmapheresis typically continues daily until antibody levels are suppressed or for 14 days. Treatment may be considered for an extended period if active pulmonary disease is apparent or the antibody level does not decline as expected. Plasmapheresis is always followed by immunosuppressive therapy—typically glucocorticoids and cyclophosphamide. Recent studies indicate the potential benefits of immunoadsorption agents as part of plasmapheresis in treating anti-GBM disease.[1][25] Typically, daily plasmapheresis is performed until anti-glomerular basement membrane antibodies are undetectable, with steroid and cyclophosphamide continuing after that for 3 to 6 months until full remission is achieved.[1][26](B3)
Many protocols recommend initiating treatment with 1 g of methylprednisolone daily for 3 days, followed by oral glucocorticoid treatment with prednisolone at a dosage of 1 mg/kg/d (up to a maximum of 60 mg) given orally. The dosage should be reduced weekly to 20 mg by 6 weeks, then gradually tapered until entirely stopped by 6 months.[1]
The dosage of cyclophosphamide is 1 to 2 mg/kg/d orally but not more than 100 mg/d for patients older than 60. The white blood cell count should be monitored and kept above 5000 cells/µL.[26] Rituximab or mycophenolate mofetil is recommended for patients experiencing adverse effects from cyclophosphamide, such as gross hematuria. Of note, rituximab is removed by plasmapheresis, especially if plasmapheresis is performed within a 3-day window after rituximab infusion, so this must be taken into account when dosing both modalities.[27][28][29](B2)
Recently, the anti-lymphocytic agent alemtuzumab has been studied to halt the progression of anti-GBM disease (although it is also thought to be a possible cause of this). Another promising medication is imlifidase, an IgG protease approved for severe anti-GBM disease. Studies show a reduction in antibody levels, but limited data are available at this time.[1] Following this remission phase, maintenance treatment with an agent associated with fewer adverse effects, such as low-dose prednisone or azathioprine, should be considered. The optimal duration of treatment is unclear. Overall, relapse remains rare.[26] (B3)
Double-Positive Antibody Crescentic Glomerulonephritis
Treatment for double-positive antibody crescentic glomerulonephritis follows the same approach as for pauci-immune glomerulonephritis, but plasmapheresis should be included. As mentioned earlier, renal manifestations follow an anti-GBM pattern, whereas systemic symptoms are similar to those of ANCA vasculitis.[30] Prolonged immunosuppression and long-term monitoring are crucial; as opposed to single-positive anti-GBM disease, relapses are not uncommon.[17] For full treatment recommendations, please see the "Double-Positive Antibody Crescentic Glomerulonephritis" section in StatPearls' companion reference, "Rapidly Progressive Glomerulonephritis."(A1)
Differential Diagnosis
All the pulmonary-renal syndromes that affect the lung and kidney should be considered in the differentials:
- Granulomatosis with polyangiitis (previously called Wegener granulomatosis)
- Microscopic polyangiitis
- Eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome)
- Systemic lupus erythematosus
Some IgA-mediated disorders also present with pulmonary-renal syndromes:
- IgA nephropathy
- IgA vasculitis (Henoch-Schönlein purpura)
Other differential diagnoses include the following:
- Acute glomerulonephritis
- Community-acquired pneumonia with infection-related glomerulonephritis
- Cryoglobulinemia
- Endocarditis
- Drug-induced vasculitis
- Alport syndrome (rarely associated with pulmonary symptoms)
Prognosis
Poor prognostic indicators include oligo- or anuria, globally sclerotic crescents, and kidney failure requiring dialysis. Fortunately, relapse or recurrent disease after kidney transplantation are both uncommon, occurring in fewer than 3% of cases. Results from one long-term study found that in patients who presented with a creatinine less than 5.7 mg/dL, 1- and 5-year survival were 95% and 94%, respectively. However, in patients presenting with a need for dialysis, only 8% recovered renal function at 1 year.[21]
Complications
Complications can be categorized into 2 forms—those arising directly from the disease and those resulting from the treatment.
Disease-Related Complications
- Pulmonary hemorrhage is often observed in cases of anti-GBM disease and can lead to mechanical ventilation (and its associated complications.)
- Renal failure can lead to dialysis and associated complications such as catheter-related infection or bleeding.
Treatment-Related Complications
The primary complications associated with immunosuppressive therapy are various opportunistic infections, which can sometimes be life-threatening. Cyclophosphamide has specific other complications, including cystitis and hematuria. Older patients are particularly prone to infections and complications arising from cyclophosphamide use.[16] Plasmapheresis is associated with removing clotting factors, increasing the patient's susceptibility to bleeding.
Consultations
A nephrologist should be consulted for the evaluation of a patient for the following:
- Differential diagnosis of renal disease
- Indication for renal biopsy
- Requirement for hemodialysis
- Plasmapheresis
As these patients may present with serious and life-threatening pulmonary complications, a pulmonologist should be consulted to guide therapy. A surgeon's assistance may also be needed when vascular access is needed for hemodialysis or plasmapheresis.
Deterrence and Patient Education
Patients should be made aware of the treatment options available and various adverse effects of medications. Patients and caregivers should be educated on when to seek medical help and on symptoms of possible relapse such as hematuria, edema, or hypertension.
Pearls and Other Issues
The prognosis for this spectrum of diseases is overall good, provided the disease is identified efficiently, and treatment started promptly. Overall survival and renal recovery track along with the degree of renal impairment on presentation. Recurrence is rare but possible, and patients typically do well with recurrent episodes; this is most likely due to heightened clinical suspicion. Treatment in recurrent cases is identical to the initial episode.[31]
Patients who do not recover renal function and remain dependent on renal replacement therapy may qualify for renal transplantation. Anti-glomerular basement membrane antibody titers should be negative for at least 6 months before transplantation.[32] These patients rarely experience recurrent disease following transplant, but it has been reported.[9] There is a theory that long-term use of immunosuppressive agents intended to protect the graft from rejection simultaneously controls the autoimmune disease.[33]
Enhancing Healthcare Team Outcomes
Patients with Goodpasture syndrome are at risk of renal failure and respiratory compromise. Early identification and management of these cases are imperative in reducing morbidity and mortality. The care of patients necessitates a collaborative approach among healthcare professionals to ensure patient-centered care and improve overall outcomes. An interprofessional team consisting of clinicians, nurses, nurse practitioners, physician assistants, and pharmacists will provide the best results. The involvement of pulmonary/critical care, nephrology, and rheumatology should be sought. Transfer to a center capable of providing input from those specialties and providing plasmapheresis is advisable. Initial lab assays should be expedited, and protocols should be implemented for all team members to be aware of positive test results. Nephrologists, pulmonologists, critical care physicians, advanced practitioners, nurses, pharmacists, and other health professionals involved in the care of these patients should possess the essential clinical skills and knowledge to diagnose and manage anti-GBM disease accurately and appropriately.
A strategic approach is equally crucial, involving evidence-based strategies to optimize treatment plans and minimize adverse events. Ethical considerations must guide decision-making, ensuring informed consent and respecting patient autonomy in treatment choices. Each healthcare professional must be aware of their responsibilities and contribute their unique expertise to the patient's care plan, fostering a multidisciplinary approach. Effective interprofessional communication is paramount, allowing seamless information exchange and collaborative decision-making among the team members. Care coordination plays a pivotal role in ensuring that the patient's journey from diagnosis to treatment and follow-up is well-managed, minimizing errors and enhancing patient safety. By embracing these principles of skill, strategy, ethics, responsibilities, interprofessional communication, and care coordination, healthcare professionals can deliver patient-centered care, ultimately improving patient outcomes and enhancing team performance in the management of Goodpasture syndrome.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Reggiani F, L'Imperio V, Calatroni M, Pagni F, Sinico RA. Goodpasture syndrome and anti-glomerular basement membrane disease. Clinical and experimental rheumatology. 2023 Apr:41(4):964-974. doi: 10.55563/clinexprheumatol/tep3k5. Epub 2023 Mar 30 [PubMed PMID: 36995324]
Ooi JD, Petersen J, Tan YH, Huynh M, Willett ZJ, Ramarathinam SH, Eggenhuizen PJ, Loh KL, Watson KA, Gan PY, Alikhan MA, Dudek NL, Handel A, Hudson BG, Fugger L, Power DA, Holt SG, Coates PT, Gregersen JW, Purcell AW, Holdsworth SR, La Gruta NL, Reid HH, Rossjohn J, Kitching AR. Dominant protection from HLA-linked autoimmunity by antigen-specific regulatory T cells. Nature. 2017 May 11:545(7653):243-247. doi: 10.1038/nature22329. Epub 2017 May 3 [PubMed PMID: 28467828]
Yang R, Cui Z, Zhao J, Zhao MH. The role of HLA-DRB1 alleles on susceptibility of Chinese patients with anti-GBM disease. Clinical immunology (Orlando, Fla.). 2009 Nov:133(2):245-50. doi: 10.1016/j.clim.2009.07.005. Epub 2009 Aug 4 [PubMed PMID: 19654074]
Peto P, Salama AD. Update on antiglomerular basement membrane disease. Current opinion in rheumatology. 2011 Jan:23(1):32-7. doi: 10.1097/BOR.0b013e328341009f. Epub [PubMed PMID: 21124085]
Level 3 (low-level) evidenceChan AL, Louie S, Leslie KO, Juarez MM, Albertson TE. Cutting edge issues in Goodpasture's disease. Clinical reviews in allergy & immunology. 2011 Oct:41(2):151-62. doi: 10.1007/s12016-010-8222-2. Epub [PubMed PMID: 21207195]
Level 3 (low-level) evidenceBatal I, Reyes DB, Popham S, Bijol V. Nodular glomerulosclerosis with anti-glomerular basement membrane-like glomerulonephritis; a distinct pattern of kidney injury observed in smokers. Clinical kidney journal. 2014 Aug:7(4):361-366 [PubMed PMID: 25349695]
Cranfield A, Mathavakkannan S. Goodpasture's disease following extracorporeal shock wave lithotripsy: a case report & literature review. Clinical case reports. 2015 Mar:3(3):160-4. doi: 10.1002/ccr3.190. Epub 2014 Dec 4 [PubMed PMID: 25838905]
Level 3 (low-level) evidenceKluth DC, Rees AJ. Anti-glomerular basement membrane disease. Journal of the American Society of Nephrology : JASN. 1999 Nov:10(11):2446-53 [PubMed PMID: 10541306]
Tang W, McDonald SP, Hawley CM, Badve SV, Boudville NC, Brown FG, Clayton PA, Campbell SB, de Zoysa JR, Johnson DW. Anti-glomerular basement membrane antibody disease is an uncommon cause of end-stage renal disease. Kidney international. 2013 Mar:83(3):503-10. doi: 10.1038/ki.2012.375. Epub 2012 Dec 19 [PubMed PMID: 23254902]
Level 2 (mid-level) evidenceJennette JC. Rapidly progressive crescentic glomerulonephritis. Kidney international. 2003 Mar:63(3):1164-77 [PubMed PMID: 12631105]
Salama AD, Levy JB, Lightstone L, Pusey CD. Goodpasture's disease. Lancet (London, England). 2001 Sep 15:358(9285):917-20 [PubMed PMID: 11567730]
Level 3 (low-level) evidencePozzi A, Yurchenco PD, Iozzo RV. The nature and biology of basement membranes. Matrix biology : journal of the International Society for Matrix Biology. 2017 Jan:57-58():1-11. doi: 10.1016/j.matbio.2016.12.009. Epub 2016 Dec 28 [PubMed PMID: 28040522]
Pedchenko V, Kitching AR, Hudson BG. Goodpasture's autoimmune disease - A collagen IV disorder. Matrix biology : journal of the International Society for Matrix Biology. 2018 Oct:71-72():240-249. doi: 10.1016/j.matbio.2018.05.004. Epub 2018 May 12 [PubMed PMID: 29763670]
Cui Z, Zhao J, Jia XY, Zhu SN, Zhao MH. Clinical features and outcomes of anti-glomerular basement membrane disease in older patients. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2011 Apr:57(4):575-82. doi: 10.1053/j.ajkd.2010.09.022. Epub 2010 Dec 18 [PubMed PMID: 21168945]
Level 2 (mid-level) evidenceAnguiano L, Kain R, Anders HJ. The glomerular crescent: triggers, evolution, resolution, and implications for therapy. Current opinion in nephrology and hypertension. 2020 May:29(3):302-309. doi: 10.1097/MNH.0000000000000596. Epub [PubMed PMID: 32132388]
Level 3 (low-level) evidenceArimura Y, Muso E, Fujimoto S, Hasegawa M, Kaname S, Usui J, Ihara T, Kobayashi M, Itabashi M, Kitagawa K, Hirahashi J, Kimura K, Matsuo S. Evidence-based clinical practice guidelines for rapidly progressive glomerulonephritis 2014. Clinical and experimental nephrology. 2016 Jun:20(3):322-41. doi: 10.1007/s10157-015-1218-8. Epub [PubMed PMID: 27099135]
Level 1 (high-level) evidenceWalsh M, Merkel PA, Peh CA, Szpirt W, Guillevin L, Pusey CD, De Zoysa J, Ives N, Clark WF, Quillen K, Winters JL, Wheatley K, Jayne D, PEXIVAS Investigators. Plasma exchange and glucocorticoid dosing in the treatment of anti-neutrophil cytoplasm antibody associated vasculitis (PEXIVAS): protocol for a randomized controlled trial. Trials. 2013 Mar 14:14():73. doi: 10.1186/1745-6215-14-73. Epub 2013 Mar 14 [PubMed PMID: 23497590]
Level 1 (high-level) evidenceMcAdoo SP, Tanna A, Hrušková Z, Holm L, Weiner M, Arulkumaran N, Kang A, Satrapová V, Levy J, Ohlsson S, Tesar V, Segelmark M, Pusey CD. Patients double-seropositive for ANCA and anti-GBM antibodies have varied renal survival, frequency of relapse, and outcomes compared to single-seropositive patients. Kidney international. 2017 Sep:92(3):693-702. doi: 10.1016/j.kint.2017.03.014. Epub 2017 May 12 [PubMed PMID: 28506760]
Zhao J, Yan Y, Cui Z, Yang R, Zhao MH. The immunoglobulin G subclass distribution of anti-GBM autoantibodies against rHalpha3(IV)NC1 is associated with disease severity. Human immunology. 2009 Jun:70(6):425-9. doi: 10.1016/j.humimm.2009.04.004. Epub 2009 Apr 11 [PubMed PMID: 19364515]
Level 3 (low-level) evidenceGluhovschi C, Gadalean F, Velciov S, Nistor M, Petrica L. Three Diseases Mediated by Different Immunopathologic Mechanisms-ANCA-Associated Vasculitis, Anti-Glomerular Basement Membrane Disease, and Immune Complex-Mediated Glomerulonephritis-A Common Clinical and Histopathologic Picture: Rapidly Progressive Crescentic Glomerulonephritis. Biomedicines. 2023 Nov 6:11(11):. doi: 10.3390/biomedicines11112978. Epub 2023 Nov 6 [PubMed PMID: 38001978]
Berden AE, Ferrario F, Hagen EC, Jayne DR, Jennette JC, Joh K, Neumann I, Noël LH, Pusey CD, Waldherr R, Bruijn JA, Bajema IM. Histopathologic classification of ANCA-associated glomerulonephritis. Journal of the American Society of Nephrology : JASN. 2010 Oct:21(10):1628-36. doi: 10.1681/ASN.2010050477. Epub 2010 Jul 8 [PubMed PMID: 20616173]
Kalluri R, Wilson CB, Weber M, Gunwar S, Chonko AM, Neilson EG, Hudson BG. Identification of the alpha 3 chain of type IV collagen as the common autoantigen in antibasement membrane disease and Goodpasture syndrome. Journal of the American Society of Nephrology : JASN. 1995 Oct:6(4):1178-85 [PubMed PMID: 8589284]
Level 3 (low-level) evidenceMcAdoo SP, Pusey CD. Anti-Glomerular Basement Membrane Disease. Clinical journal of the American Society of Nephrology : CJASN. 2017 Jul 7:12(7):1162-1172. doi: 10.2215/CJN.01380217. Epub 2017 May 17 [PubMed PMID: 28515156]
Heitz M, Carron PL, Clavarino G, Jouve T, Pinel N, Guebre-Egziabher F, Rostaing L. Use of rituximab as an induction therapy in anti-glomerular basement-membrane disease. BMC nephrology. 2018 Sep 20:19(1):241. doi: 10.1186/s12882-018-1038-7. Epub 2018 Sep 20 [PubMed PMID: 30236081]
Greenhall GH, Salama AD. What is new in the management of rapidly progressive glomerulonephritis? Clinical kidney journal. 2015 Apr:8(2):143-50. doi: 10.1093/ckj/sfv008. Epub 2015 Feb 19 [PubMed PMID: 25815169]
Syeda UA, Singer NG, Magrey M. Anti-glomerular basement membrane antibody disease treated with rituximab: A case-based review. Seminars in arthritis and rheumatism. 2013 Jun:42(6):567-72. doi: 10.1016/j.semarthrit.2012.10.007. Epub 2013 Jan 24 [PubMed PMID: 23352254]
Level 3 (low-level) evidenceLevy JB, Turner AN, Rees AJ, Pusey CD. Long-term outcome of anti-glomerular basement membrane antibody disease treated with plasma exchange and immunosuppression. Annals of internal medicine. 2001 Jun 5:134(11):1033-42 [PubMed PMID: 11388816]
Level 2 (mid-level) evidenceApaydin S. The treatment of ANCA-associated rapidly-progressive glomerulonephritis and Goodpasture syndrome with therapeutic apheresis. Transfusion and apheresis science : official journal of the World Apheresis Association : official journal of the European Society for Haemapheresis. 2018 Feb:57(1):8-12. doi: 10.1016/j.transci.2018.02.007. Epub 2018 Feb 20 [PubMed PMID: 29503131]
Puisset F, White-Koning M, Kamar N, Huart A, Haberer F, Blasco H, Le Guellec C, Lafont T, Grand A, Rostaing L, Chatelut E, Pourrat J. Population pharmacokinetics of rituximab with or without plasmapheresis in kidney patients with antibody-mediated disease. British journal of clinical pharmacology. 2013 Nov:76(5):734-40. doi: 10.1111/bcp.12098. Epub [PubMed PMID: 23432476]
Pacheco M, Silva JE, Silva C, Soares N, Almeida J. Double-Positive Anti-GBM and ANCA-MPO Vasculitis Presenting With Crescentic Glomerulonephritis. Cureus. 2021 May 2:13(5):e14806. doi: 10.7759/cureus.14806. Epub 2021 May 2 [PubMed PMID: 34094762]
Levy JB, Lachmann RH, Pusey CD. Recurrent Goodpasture's disease. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1996 Apr:27(4):573-8 [PubMed PMID: 8678069]
Level 3 (low-level) evidenceBeck L, Bomback AS, Choi MJ, Holzman LB, Langford C, Mariani LH, Somers MJ, Trachtman H, Waldman M. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for glomerulonephritis. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013 Sep:62(3):403-41. doi: 10.1053/j.ajkd.2013.06.002. Epub 2013 Jul 18 [PubMed PMID: 23871408]
Level 2 (mid-level) evidenceSauter M, Schmid H, Anders HJ, Heller F, Weiss M, Sitter T. Loss of a renal graft due to recurrence of anti-GBM disease despite rituximab therapy. Clinical transplantation. 2009 Jan-Feb:23(1):132-6. doi: 10.1111/j.1399-0012.2008.00912.x. Epub 2008 Dec 4 [PubMed PMID: 19087095]
Level 3 (low-level) evidence