Introduction
EEG activity reflects the temporal summation of the synchronous activity of millions of cortical neurons that are spatially aligned. Analyzing and interpreting the EEG is both an art and science. The normal EEG is extremely diverse and has a broad range of physiological variability.
Technique or Treatment
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Technique or Treatment
It is extremely important to follow a systematic approach while interpreting the waveforms in an EEG recording. Even before one starts the analysis, you need to have information regarding several confounding variables including patient's age, state of consciousness, physical and mental activity and the presence of different biological, environmental stimuli and pharmacological agents which can affect the waveforms.
Clinical Significance
EEG waveforms may be characterized based on their location, amplitude, frequency, morphology, continuity (rhythmic, intermittent or continuous), synchrony, symmetry, and reactivity. However, the most frequently used method to classify EEG waveforms is by the frequency, so much so, that EEG waves are named based on their frequency range using Greek numerals. The most commonly studied waveforms include delta (0.5 to 4Hz); theta (4 to 7Hz); alpha (8 to 12Hz); sigma (12 to 16Hz) and beta (13 to 30Hz). In addition, there are other waveforms such as infra slow oscillations (ISO) (less than 0.5Hz) and high-frequency oscillations (HFOs) (greater than 30Hz) which are outside the conventional bandwidth of clinical EEG but have recently found clinical importance with the advent of digital signal processing.
Frequency:
The conventional bandwidth of clinical EEG focuses on the analysis of waveforms ranging from 0.5Hz to 70Hz. This analysis occurs through the use of bandpass filtering of the EEG recordings. However, a broader EEG bandwidth has undergone examination by clinical neurophysiologists and researchers and has been found to be clinically significant in certain conditions. Elimination of the lower (infra-slow) or higher (ultra-fast) bands of the EEG frequency spectrum in routine EEG results in loss of several important physiological and pathological meaningful features of brain activity. A full bandwidth EEG (FbEEG) looks at the full, physiologically and clinically relevant waveforms without any trade-off that would favor one frequency band at the expense of another.[1] However, recording EEG data at extremely high frequencies is not done routinely in clinical practice because it requires special equipment that can acquire data at higher sampling frequencies which in turn increases the space needed to store this information. Based on the FbEEG recording, EEG waveforms can be characterized by various types:-
1. Infra-slow oscillations (ISO) (less than 0.5Hz): ISOs are the dominating frequency in the preterm neonates is as low as 0.01 to 0.1 Hz and are termed as spontaneous activity transients (SAT). SATs represent endogenously driven, the spontaneous activity which is crucial in shaping neuronal connectivity at an early immature stage where sensory input has little or no role at all.[2] Additionally, ISOs at a wide range of frequencies (0.02 to 0.2 Hz) are also present during non-REM sleep, phase synchronized with higher frequency EEG activities.[3]
Most of the research on low-frequency EEG has focused on various kinds of cognitive tasks and states such as contingent stimulation (contingent negative variation, CNV); motor movements (Bereitschafts potential) and the orienting paradigm.[4][5] Duration of these slow scalp-recorded potentials are up to several seconds, and often an amplitude in the order of only a few microvolts, hence requiring FbEEG as well as electrodes and skin–electrodecontacts with genuine DC properties for their accurate recording.
Finally, invasive/non-invasive EEG monitoring in animal models and humans have established that seizures have associations with very slow EEG responses along with variable low-frequency fluctuations at the seizure focus.[6] Very recently, non-invasive ictal DC recordings have demonstrated that focal onset seizures correlate with long and relatively high amplitude DC shifts.[7]
2. Delta (0.5 to 4Hz): Delta rhythm is physiologically seen in deep sleep and is prominent in the frontocentral head regions. Pathological delta rhythm presents in awake states in case of generalized encephalopathy and focal cerebral dysfunction. Frontal intermittent rhythmic delta activity (FIRDA) presents in adults,[8] whereas occipital intermittent rhythmic delta activity (OIRDA) occurs in children.[9] Temporal intermittent rhythmic delta activity (TIRDA) is frequently seen in individuals who have temporal lobe epilepsy.[10]
3. Theta (4 to 7Hz): This is the rhythm which is brought on by drowsiness as well as early stages of sleep such as N1 and N2. It is most prominent in the fronto-central head regions and slowly migrates backward replacing the alpha rhythm due to early drowsiness. Heightened emotional states can also enhance frontal rhythmic theta rhythm in children and young adults. Focal theta activity during awake states is suggestive of focal cerebral dysfunction.
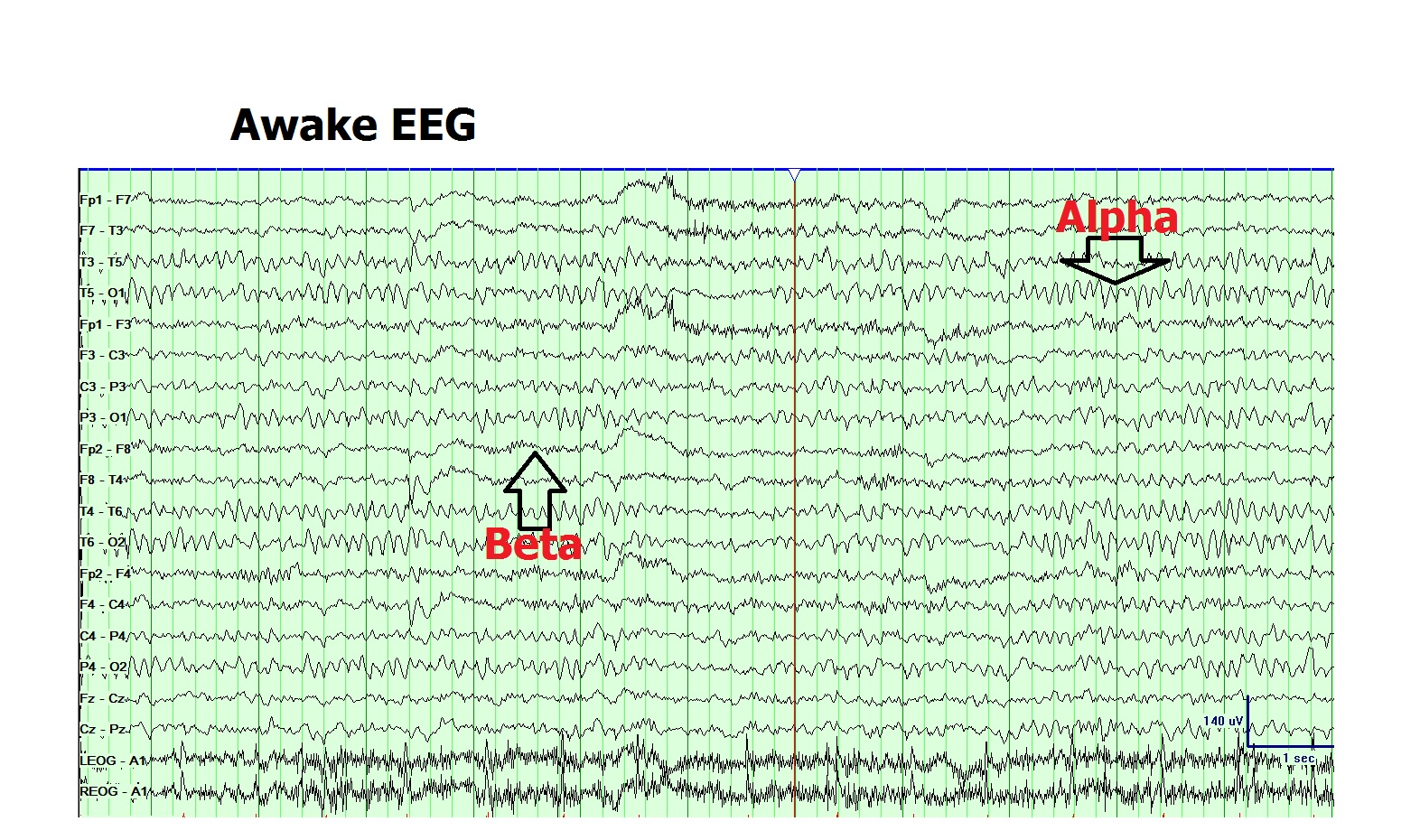
4. Alpha (8 to 12Hz): The posterior dominant alpha rhythm is characteristically present in normal awake EEG recordings in the occipital head region. It is the defining feature of the normal background rhythm of the adult EEG recording. The posterior rhythm attains the alpha range of 8Hz at the age of 3 years and does not decline even until the ninth decade of life in healthy individuals. Fast variants of background alpha rhythm are seen in the normal population. Slowing of the background alpha rhythm is considered to be a sign of generalized cerebral dysfunction.[11] The amplitude of alpha rhythm varies in different individuals as well as at different times in the same individual. Reactivity of the alpha rhythm is characteristic and helps in its recognition. It is best seen with the eyes closed and during mental relaxation and is characteristically attenuated by eye-opening and mental effort. In diffuse encephalopathy, patients may portray generalized-alpha activity which is non-reactive to internal or external stimuli and goes by the name of "alpha coma."
Mu rhythm is another type of alpha rhythm which presents in the central head regions, and they have an arch like morphology. This rhythm characteristically disappears with the motor activity of the contralateral limbs or thinking about initiating motor activity.[12] However, It is relatively unchanged with eye-opening. They are frequently seen in young adults and are not as common in children and the elderly. Attenuating factors include fatigue, somatosensory stimulation, and mental arithmetic. They are quite asymmetric and asynchronous on the two sides.
5. Sigma waves: This activity is seen physiologically in N2 sleep and is called sleep spindles or sigma waves. They may be slow (12 to 14Hz) or fast (14 to 16Hz) and are seen most prominently in the fronto-central head regions.[13] Pathological spindle rhythm can be seen in generalized encephalopathy and is known as "spindle coma."
6. Beta (13 to 30Hz): Beta rhythm is the most frequently seen rhythm in normal adults and children. It is most prominent in the frontal and central head regions and attenuates as it goes posteriorly. The amplitude of beta activity is usually 10 to 20 microvolts, which seldom increase above 30 microvolts. It often increases in amplitude during drowsiness, N1 sleep and subsequently decreases in N2 & N3 sleep. Most of the sedative medications such as barbiturates, chloral hydrate, and benzodiazepines increase the amplitude and quantity of beta activity in individuals.[14] Focal, regional or hemispheric attenuation of beta can occur with a cortical injury, malformations, subdural, epidural or subgaleal fluid collections.
7. High-Frequency Oscillations (HFOs) (greater than 30Hz): These further classify as gamma (30 to 80Hz); ripples (80 to 200Hz) & fast ripples (200 t o500Hz). Gamma rhythm has been attributed to sensory perception integrating different areas. There has been extensive research worldwide on HFOs particularly in relation with epilepsy. Epileptic foci are known to generate episodes of very high-frequency activity. Intracranial depth recordings from the epileptic hippocampus (animal and human models) have reported ultrafast frequency bursts (fast ripples), which probably correlate with the local epileptogenicity of the brain tissue.[15] On the other hand, subdural recordings during presurgical evaluation of epilepsy have demonstrated that activity bursts at a relatively lower frequency range (60 to 100 Hz) may likewise indicate the location of an epileptic focus. There is evidence for interictal HFOs as possible biomarkers of human epileptogenic brain tissue.
Ultrafast EEG activity correlates to cognitive states and event-related potentials. The importance of gamma rhythms in a large variety of cognitive functions has been well established.[16][17] Brain stem evoked potentials (BERA) is a well established and routinely measured category of ultrafast EEG signals. There are reports of HFOs (greater than 200 Hz) related to somatosensory stimulation or motor movements and their sensitivity to vigilance states, motor interference or pharmacological manipulations, such as anesthetics or sedatives offer newer options for brain monitoring and diagnostics.[18][19] They may help in early detection of demyelination and other disorders of cortical integrity.
Morphology:
EEG Transients are isolated waveforms or complexes that are distinguishable from background activity. Several EEG transients present in normal individuals which are benign and need to be differentiated from pathological transients. Identification of these waveforms as non-epileptic requires training and experience. Misinterpretation of non-epileptiform transients will lead to overdiagnosis of epilepsy, unnecessary prolonged treatments with antiepileptic drugs and other medico-legal consequences.
Identification of Non-epileptiform transients
Nonepileptiform transients are waveforms that have an epileptiform appearance but have no relation with epileptic seizures.[20] They may be sharply contoured and can occur as isolated arrhythmic bursts. Researchers have observed that most of the common non-epileptic transients occur during drowsiness and light sleep.[21] We will describe some of these commonly observed non-epileptiform transients.
1. Lambda waves: Lambda waves are positive sharp transients occurring in the occipital head region in awake states and occur most prominently during visual exploration and usually disappears on eye closure.[22]
2. Positive Occipital Sharp Transients of Sleep (POSTS): POSTS are positive sharp transients resembling lambda waves and are present in about 50 to 80% of healthy individuals during NREM sleep. They are most frequently observed in adolescents and young adults and are highest during the initial phase of drowsiness as opposed to deeper stages of NREM sleep.[23]
3. 6 Hz Spike and wave (phantom spike and wave): These are low amplitude, poorly discernible spikes that occur within a repeating spike and slow wave complex. They are characteristically 5 to 6Hz with amplitudes of under 40 microvolts and duration of spike shorter than 30 milliseconds.[24] They may have either a frontal or occipital predominance and seen commonly in adolescents and young adults.
4. 14 & 6Hz Positive Spikes (Ctenoids): These are unilateral, bi-synchronous or asynchronous, regular repetitions with arciform morphology that are centered in the posterior temporal region and occur with broad distribution. They are frequently seen during drowsiness and light sleep.[25]
5. Vertex Sharp Transients (VSTs): VSTs appear as mono or diphasic and often triphasic waves and surface negative sharp wave with phase reversal at or near the vertex and occurs in drowsiness and NREM sleep. They are typically 100 milliseconds in duration.
6. K Complex: These are polyphasic waves which are longer than 0.5 sec in duration, less sharply contoured and are often followed by sleep spindles.[26]
7. Benign Epileptiform Transients of Sleep (BETS) or Small Sharp Spikes (SSS) or Benign Sporadic Sleep Spike (BSSS): These are low amplitude, sharply contoured monophasic or diphasic transients occurring most frequently in N1 and N2 sleep. Most likely to occur in adults between 30 and 60 years. It has amplitudes of under 90 microvolts and duration of longer than 90 milliseconds. It is seen appearing most frequently in the mid-temporal region with a broad field extending into the adjacent frontal region.
8. Wicket Waves (Wicket Rhythm): These are commonly seen EEG transients that are monophasic, sharply contoured with symmetric upgoing and downgoing phase. They typically arise from ongoing background activity and do not disrupt the background.[27] It is present in relaxed wakefulness and facilitated by drowsiness and most commonly present in mid-adulthood or older adults
9. Rhythmic Mid-temporal Theta of Drowsiness (RMTD): Previously known as Psychomotor Variant: RMTDs are trains of theta activity that are seen during the sleep-wake transition and are centered around the mid-temporal region and may spread to the anterior and posterior temporal as well as posterior parietal regions. They have a monomorphic pattern with a sharp or notched contour which is very characteristic.[28]
10. Subclinical Rhythmic Electroencephalographic Discharges of Adults (SREDA): This is an EEG pattern which has an unclear clinical significance but is frequently diagnosed as an epileptiform pattern. The onset of this rhythm can be sudden or widespread and may evolve from slower delta to faster theta rhythm. It is seen usually during wakefulness or light sleep and may sometimes be activated by hyperventilation. They may be widespread but usually maximal over the parietal and posterior temporal regions and are almost always bilaterally synchronous and symmetric.[29] They may last anywhere from 10 seconds to 5 minutes with an average of 40 to 80 seconds with either abrupt or gradual resolution.
Enhancing Healthcare Team Outcomes
It is essential for the beginner to become well versed with the identification of normal EEG variations before interpreting waveforms that are pathological. On the other hand, a single normal EEG recording does not exclude the presence of pathology because electrographic phenomena are often transient. an interprofessional team approach, including physicians, nurses, and mid-level providers, correctly trained in the discipline of EEG interpretation and subsequent treatment, can work together to achieve the best outcome for the patient. [Level V]
Media
References
Vanhatalo S, Voipio J, Kaila K. Full-band EEG (FbEEG): an emerging standard in electroencephalography. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2005 Jan:116(1):1-8 [PubMed PMID: 15589176]
Vanhatalo S, Tallgren P, Andersson S, Sainio K, Voipio J, Kaila K. DC-EEG discloses prominent, very slow activity patterns during sleep in preterm infants. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2002 Nov:113(11):1822-5 [PubMed PMID: 12417237]
Vanhatalo S, Palva JM, Holmes MD, Miller JW, Voipio J, Kaila K. Infraslow oscillations modulate excitability and interictal epileptic activity in the human cortex during sleep. Proceedings of the National Academy of Sciences of the United States of America. 2004 Apr 6:101(14):5053-7 [PubMed PMID: 15044698]
Birbaumer N, Elbert T, Canavan AG, Rockstroh B. Slow potentials of the cerebral cortex and behavior. Physiological reviews. 1990 Jan:70(1):1-41 [PubMed PMID: 2404287]
Level 3 (low-level) evidenceCui RQ, Huter D, Egkher A, Lang W, Lindinger G, Deecke L. High resolution DC-EEG mapping of the Bereitschaftspotential preceding simple or complex bimanual sequential finger movement. Experimental brain research. 2000 Sep:134(1):49-57 [PubMed PMID: 11026725]
Ikeda A, Terada K, Mikuni N, Burgess RC, Comair Y, Taki W, Hamano T, Kimura J, Lüders HO, Shibasaki H. Subdural recording of ictal DC shifts in neocortical seizures in humans. Epilepsia. 1996 Jul:37(7):662-74 [PubMed PMID: 8681899]
Level 3 (low-level) evidenceVoipio J, Tallgren P, Heinonen E, Vanhatalo S, Kaila K. Millivolt-scale DC shifts in the human scalp EEG: evidence for a nonneuronal generator. Journal of neurophysiology. 2003 Apr:89(4):2208-14 [PubMed PMID: 12612037]
CORDEAU JP. Monorhythmic frontal delta activity in the human electroencephalogram: a study of 100 cases. Electroencephalography and clinical neurophysiology. 1959 Nov:11():733-46 [PubMed PMID: 13811933]
Level 3 (low-level) evidenceDalby MA. Epilepsy and 3 per second spike and wave rhythms. A clinical, electroencephalographic and prognostic analysis of 346 patients. Acta neurologica Scandinavica. 1969:():Suppl 40:3+ [PubMed PMID: 4979890]
Reiher J, Beaudry M, Leduc CP. Temporal intermittent rhythmic delta activity (TIRDA) in the diagnosis of complex partial epilepsy: sensitivity, specificity and predictive value. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 1989 Nov:16(4):398-401 [PubMed PMID: 2804800]
AIRD RB, GASTAUT Y. Occipital and posterior electroencephalographic rhythms. Electroencephalography and clinical neurophysiology. 1959 Nov:11():637-56 [PubMed PMID: 13792196]
CHATRIAN GE, PETERSEN MC, LAZARTE JA. The blocking of the rolandic wicket rhythm and some central changes related to movement. Electroencephalography and clinical neurophysiology. 1959 Aug:11(3):497-510 [PubMed PMID: 13663823]
Gondeck AR, Smith JR. Dynamics of human sleep sigma spindles. Electroencephalography and clinical neurophysiology. 1974 Sep:37(3):293-7 [PubMed PMID: 4136646]
Frost JD Jr, Carrie JR, Borda RP, Kellaway P. The effects of dalmane (flurazepam hydrochloride) on human EEG characteristics. Electroencephalography and clinical neurophysiology. 1973 Feb:34(2):171-5 [PubMed PMID: 4119530]
Worrell G, Gotman J. High-frequency oscillations and other electrophysiological biomarkers of epilepsy: clinical studies. Biomarkers in medicine. 2011 Oct:5(5):557-66. doi: 10.2217/bmm.11.74. Epub [PubMed PMID: 22003904]
Ward LM. Synchronous neural oscillations and cognitive processes. Trends in cognitive sciences. 2003 Dec:7(12):553-9 [PubMed PMID: 14643372]
Tallon-Baudry C. Oscillatory synchrony and human visual cognition. Journal of physiology, Paris. 2003 Mar-May:97(2-3):355-63 [PubMed PMID: 14766151]
Level 3 (low-level) evidenceHaueisen J, Heuer T, Nowak H, Liepert J, Weiller C, Okada Y, Curio G. The influence of lorazepam on somatosensory-evoked fast frequency (600 Hz) activity in MEG. Brain research. 2000 Aug 18:874(1):10-4 [PubMed PMID: 10936218]
Klostermann F, Gobbele R, Buchner H, Siedenberg R, Curio G. Differential gating of slow postsynaptic and high-frequency spike-like components in human somatosensory evoked potentials under isometric motor interference. Brain research. 2001 Dec 13:922(1):95-103 [PubMed PMID: 11730706]
Klass DW, Westmoreland BF. Nonepileptogenic epileptiform electroencephalographic activity. Annals of neurology. 1985 Dec:18(6):627-35 [PubMed PMID: 4083847]
Westmoreland BF, Klass DW. Unusual EEG patterns. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 1990 Apr:7(2):209-28 [PubMed PMID: 2187021]
Scott DF, Bickford RG. Electrophysiologic studies during scanning and passive eye movements in humans. Science (New York, N.Y.). 1967 Jan 6:155(3758):101-2 [PubMed PMID: 6015559]
Egawa I, Yoshino K, Hishikawa Y. Positive occipital sharp transients in the human sleep EEG. Folia psychiatrica et neurologica japonica. 1983:37(1):57-65 [PubMed PMID: 6884913]
Hughes JR. Two forms of the 6/sec spike and wave complex. Electroencephalography and clinical neurophysiology. 1980 May:48(5):535-50 [PubMed PMID: 6153962]
MILLEN FJ, WHITE B. Fourteen and six per second positive spike activity in children. Neurology. 1954 Jul:4(7):541-9 [PubMed PMID: 13176673]
PAMPIGLIONE C. The phenomenon of adaptation in human E.E.G.; a study of K complexes. Revue neurologique. 1952:87(2):197-8 [PubMed PMID: 13014782]
Reiher J, Lebel M. Wicket spikes: clinical correlates of a previously undescribed EEG pattern. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 1977 Feb:4(1):39-47 [PubMed PMID: 837263]
Lipman IJ, Hughes JR. Rhythmic mid-temporal discharges. An electro-clinical study. Electroencephalography and clinical neurophysiology. 1969 Jul:27(1):43-7 [PubMed PMID: 4182889]
Brigo F, Ausserer H, Nardone R, Tezzon F, Manganotti P, Bongiovanni LG. Subclinical rhythmic electroencephalogram discharge of adults occurring during sleep: a diagnostic challenge. Clinical EEG and neuroscience. 2013 Jul:44(3):227-31. doi: 10.1177/1550059412466549. Epub 2013 Mar 26 [PubMed PMID: 23536379]
Level 3 (low-level) evidence