Introduction
Connective tissue is the most abundant and diverse type of animal tissue. Animal tissue divides into 4 basic groups, which include epithelial tissue, muscle tissue, nervous tissue, and connective tissue. Like a house's framework, connective tissue provides structure, support, and protection throughout the human body.[1]
Cellular Level
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Cellular Level
Connective tissue is an umbrella that encompasses a variety of tissue types, including loose and dense connective tissue, adipose, cartilage, bone, and blood. Although connective tissue is diverse, all connective tissue consists of 3 main components:
- Ground substance
- Fibers
- Cells
The ground substance and fibers comprise the extracellular matrix, the structural support of surrounding cells throughout the body. The composition of the extracellular matrix varies tremendously from organ to organ, which allows for diverse types of connective tissue.[2]
Ground substance is an amorphous gelatinous material with a high water content that occupies the space between cells and fibers. It comprises glycosaminoglycans, particularly hyaluronic acid, proteoglycans, and cell adhesion proteins, such as laminin and fibronectin, to act as a glue for cells in the extracellular matrix. The purpose of the ground substance is to allow for the exchange of cellular nutrients between cells and capillaries.[3]
Fibers are another main component of connective tissue found in the extracellular matrix. Three basic fiber types are present in all connective tissue. The amount of each type generally reflects the function and classification of the particular tissue. The 3 fiber types are:
- Collagenous fibers: large, strong fibers, mostly commonly Type I collagen, that provide high tensile strength to the extracellular matrix, found in dense and loose connective tissue.[1]
- Reticular fibers: delicate, thin fibers composed of Type III collagen that cross-link to form a supporting meshwork in the reticular lamina of the basement membrane found in soft tissues such as the liver, bone marrow, spleen, and lymph nodes.[4]
- Elastic fibers: thin, branching fibers made of elastin that provide stretch and recoil to the extracellular matrix, found in tissues such as the aorta, lung, skin, and vocal cords.[5]
To further understand the physiology of connective tissue, it is essential to note the different types of collagen, which are generally composed of fibers found in the extracellular matrix. Collagen is the most abundant protein in the human body and has 28 types.[6] The 4 most common types are:
- Type I: the most common type; flexible, strong, provides resistance to force, tension, and stretch; found in all connective tissue, notably scar tissue, tendons, ligaments, bone, cornea, skin, and dentin.
- Type II: provides resistance to pressure, found in articular and hyaline cartilage of joints and intervertebral discs.
- Type III: provides a flexible meshwork for cellular support, the main component of reticular fibers, often found in organs such as skin and blood vessels. Also abundant during the early stages of wound healing and plays a role in granulation tissue formation.[7]
- Type IV: A meshwork that provides support and attachment to the underlying extracellular matrix forms the basal lamina of the basement membrane, an essential component of the kidneys, inner ear, and eye lens.
Commons cells of the connective tissue consist of fibroblasts, macrophages, adipocytes, leukocytes, and mast cells.[8]
Collagen Synthesis and Structure
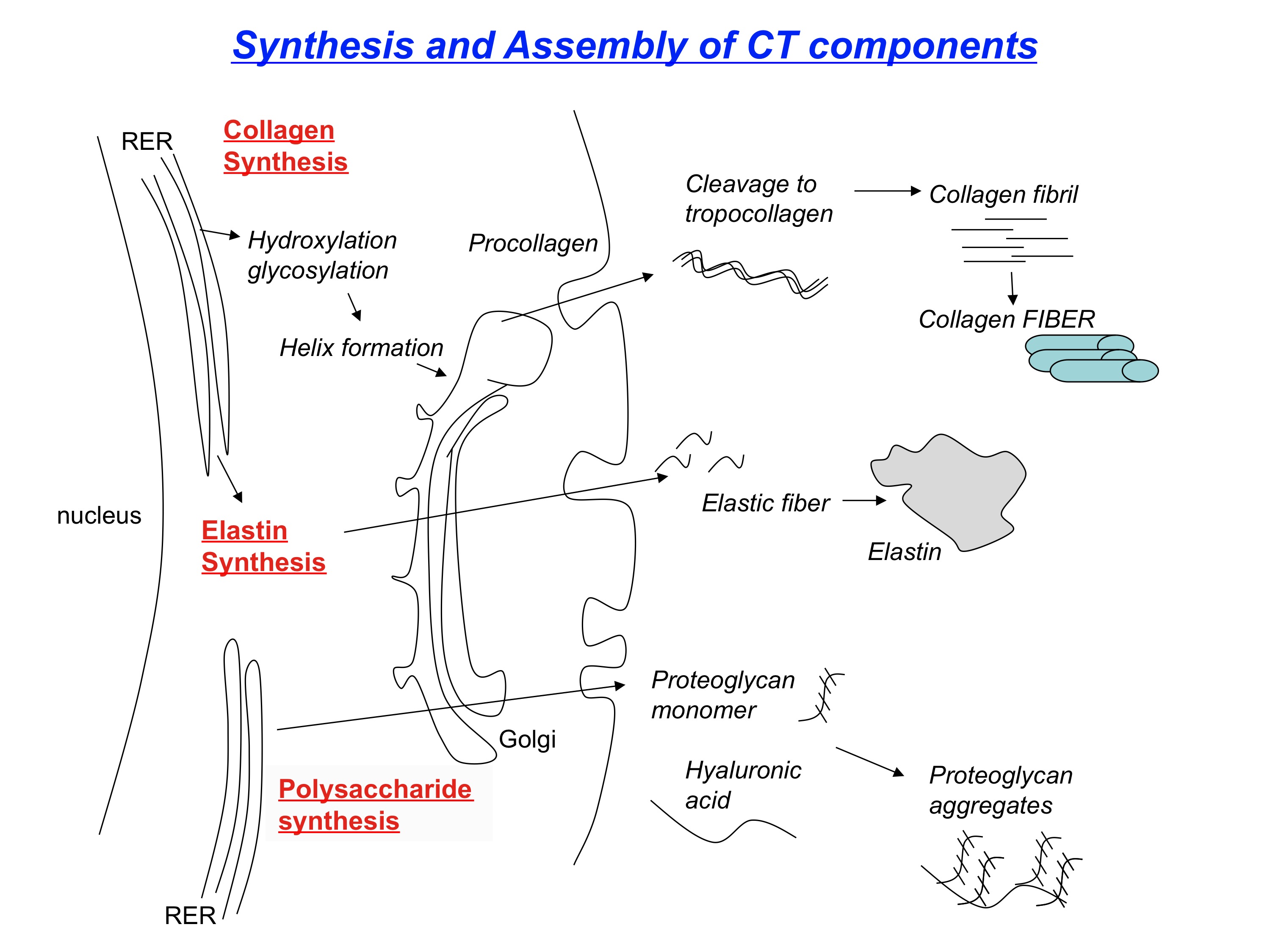
The synthesis of collagen begins with DNA translation of a polypeptide chain in the rough endoplasmic reticulum of intracellular space (See Figure. Review of Collagen Synthesis). This polypeptide chain is called preprocollagen, composed of alpha chains with repeating amino acid glycine-X-Y sequences (X and Y are proline or lysine).[9]
The proline and lysine residues of the alpha chain become hydroxylated with the help of vitamin C-dependent hydroxylases; hydroxylation helps form a stable structure. Vitamin C deficiency results in impaired hydroxylation of pre-procollagen and defective collagen synthesis, a condition known as scurvy.[10]
Next, the hydroxylated lysine of the alpha chain undergoes glycosylation with the addition of a carbohydrate. After glycosylation, the formation of disulfide bonds and hydrogen bonds between 3 different alpha chains forms a triple helix. The resultant structure is now procollagen. Defective triple helix formation leads to osteogenesis imperfecta and impaired bone matrix synthesis.[11]
Procollagen then shuttles to the extracellular space through exocytosis. Once in the extracellular space, it is cleaved at the C-terminus and N-terminus, forming what is now called tropocollagen. As a result of the cleavage, tropocollagen is insoluble in water.
Next, tropocollagen is reinforced with many adjacent tropocollagen molecules by covalent cross-linking of hydroxylated lysine residues, resulting in collagen fibrils. The cross-linking of hydroxylysine residues is made possible through a copper-dependent lysyl oxidase enzyme. Finally, several collagen fibrils accumulate to form a thick bundle; the end product is collagen fibers.[12]
Development
Connective tissue largely derives from mesenchyme, which originates from the mesoderm. However, some bones of the face and skull also have contributions from neural crest cells, which originate from the ectoderm.[13]
Function
Connective tissue has a variety of different functions, depending on its classification.[14] In summary, connective tissue provides:
- Resistance to stretch and tear
- Structural support
- Insulation
- Storage of body fuels
- A medium for intercellular exchange
Clinical Significance
There are over 200 documented disorders of connective tissue.[15] The etiology can include autoimmune diseases, genetic disorders, or cancers. One of the more common autoimmune connective tissue disorders is rheumatoid arthritis. Rheumatoid arthritis is a chronic inflammatory disorder characterized by autoimmune destruction and deterioration of cartilage and bone. It classically presents middle-aged women with symmetrical pain and swelling of the metacarpophalangeal joints and proximal interphalangeal joints with sparing of the distal interphalangeal joints. It can also manifest as low-grade fevers, myalgias, malaise, and night sweats and can affect various organs throughout the body.[16]
Osteogenesis imperfecta, also known as brittle bone disease, is a rare yet interesting inherited connective tissue disorder. It results from an autosomal dominant mutation leading to defective type I collagen synthesis and impaired bone matrix formation. It can present as recurrent fractures (notably long bones or ribs) with minimal trauma in children, which often gets mistaken as child abuse. Some other manifestations include blue sclerae, progressive hearing loss, and brittle, opalescent teeth.[17] Another example of an inherited connective tissue disease is Alport syndrome. Alport syndrome is commonly an X-linked dominant disorder resulting in a genetic defect in type IV collagen. This leads to a characteristic splitting of the glomerular basement membrane, most notably in the kidneys. This condition usually presents in boys with glomerulonephritis, sensorineural deafness, and ocular abnormalities such as retinopathy and lens dislocation.[18]
One fascinating area of research that can make a significant impact in the field of orthopedics is the concept of tissue engineering. Biomedical engineers and orthopedic surgeons work together to create novel strategies to repair and regenerate damaged tissues. For example, research is being done at the University of Pennsylvania using animal meniscus as a model system to study an engineered implantable platform for wound healing. The meniscus, composed of dense connective tissue, is generally too dense to allow stem cells to move to the injury site and start the repair process. Researchers at Penn have developed a microscopic scaffold that is laced with an enzyme to loosen the matrix in addition to growth factors that attract stem cells. When the researchers placed this microscopic scaffold in damaged meniscus tissue from a cow, the matrix loosened, allowing bone stem cells to travel to the scaffold and start the repair process. The next research phase intends to look at large animal models and, eventually, human trials.[19]
Ascorbic acid (Vitamin C) is essential for activating the enzyme prolyl hydroxylase, which promotes the hydroxylation step in forming the hydroxyproline, an integral constituent of collagen. Without ascorbic acid, the collagen fibers in virtually all body tissues are defective and weak. Therefore, this vitamin is important for the growth and strength of the fibers in subcutaneous tissue, cartilage, bone, and teeth. Particularly deficiency of the ascorbic acid for about 20-30 weeks can cause scurvy. One of the most important effects of scurvy is the failure of wounds to heal.[20]
Media
(Click Image to Enlarge)
References
Kannus P. Structure of the tendon connective tissue. Scandinavian journal of medicine & science in sports. 2000 Dec:10(6):312-20 [PubMed PMID: 11085557]
Halper J, Kjaer M. Basic components of connective tissues and extracellular matrix: elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Advances in experimental medicine and biology. 2014:802():31-47. doi: 10.1007/978-94-007-7893-1_3. Epub [PubMed PMID: 24443019]
Level 3 (low-level) evidenceYoung RA. The Ground Substance of Connective Tissue. The Journal of physiology. 1894 May 29:16(5-6):325-50 [PubMed PMID: 16992170]
Hayakawa M, Kobayashi M, Hoshino T. Microfibrils: a constitutive component of reticular fibers in the mouse lymph node. Cell and tissue research. 1990 Oct:262(1):199-201 [PubMed PMID: 2257612]
Level 3 (low-level) evidenceUitto J. Biochemistry of the elastic fibers in normal connective tissues and its alterations in diseases. The Journal of investigative dermatology. 1979 Jan:72(1):1-10 [PubMed PMID: 368254]
Ricard-Blum S. The collagen family. Cold Spring Harbor perspectives in biology. 2011 Jan 1:3(1):a004978. doi: 10.1101/cshperspect.a004978. Epub 2011 Jan 1 [PubMed PMID: 21421911]
Level 3 (low-level) evidenceEhrlich HP, Krummel TM. Regulation of wound healing from a connective tissue perspective. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 1996 Apr-Jun:4(2):203-10 [PubMed PMID: 17177814]
Level 3 (low-level) evidenceMescher AL. Macrophages and fibroblasts during inflammation and tissue repair in models of organ regeneration. Regeneration (Oxford, England). 2017 Apr:4(2):39-53. doi: 10.1002/reg2.77. Epub 2017 Jun 6 [PubMed PMID: 28616244]
Pinnell SR. Regulation of collagen synthesis. The Journal of investigative dermatology. 1982 Jul:79 Suppl 1():73s-76s [PubMed PMID: 7086193]
Level 3 (low-level) evidenceLykkesfeldt J, Michels AJ, Frei B. Vitamin C. Advances in nutrition (Bethesda, Md.). 2014 Jan 1:5(1):16-8. doi: 10.3945/an.113.005157. Epub 2014 Jan 1 [PubMed PMID: 24425716]
Level 3 (low-level) evidenceMarini JC, Forlino A, Bächinger HP, Bishop NJ, Byers PH, Paepe A, Fassier F, Fratzl-Zelman N, Kozloff KM, Krakow D, Montpetit K, Semler O. Osteogenesis imperfecta. Nature reviews. Disease primers. 2017 Aug 18:3():17052. doi: 10.1038/nrdp.2017.52. Epub 2017 Aug 18 [PubMed PMID: 28820180]
Last JA, Reiser KM. Collagen biosynthesis. Environmental health perspectives. 1984 Apr:55():169-77 [PubMed PMID: 6428877]
Level 3 (low-level) evidenceOgawa M, Larue AC, Watson PM, Watson DK. Hematopoietic stem cell origin of connective tissues. Experimental hematology. 2010 Jul:38(7):540-7. doi: 10.1016/j.exphem.2010.04.005. Epub 2010 Apr 20 [PubMed PMID: 20412832]
Level 3 (low-level) evidenceShekhter AB. Connective tissue as an integral system: role of cell-cell and cell-matrix interactions. Connective tissue research. 1986:15(1-2):23-31 [PubMed PMID: 2944698]
Level 3 (low-level) evidenceGaubitz M. Epidemiology of connective tissue disorders. Rheumatology (Oxford, England). 2006 Oct:45 Suppl 3():iii3-4 [PubMed PMID: 16987829]
Heidari B. Rheumatoid Arthritis: Early diagnosis and treatment outcomes. Caspian journal of internal medicine. 2011 Winter:2(1):161-70 [PubMed PMID: 24024009]
van Dijk FS, Cobben JM, Kariminejad A, Maugeri A, Nikkels PG, van Rijn RR, Pals G. Osteogenesis Imperfecta: A Review with Clinical Examples. Molecular syndromology. 2011 Dec:2(1):1-20 [PubMed PMID: 22570641]
Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A, Kashtan CE. Alport Syndrome. GeneReviews(®). 1993:(): [PubMed PMID: 20301386]
Loebel C, Burdick JA. Engineering Stem and Stromal Cell Therapies for Musculoskeletal Tissue Repair. Cell stem cell. 2018 Mar 1:22(3):325-339. doi: 10.1016/j.stem.2018.01.014. Epub 2018 Feb 8 [PubMed PMID: 29429944]
Grosso G, Bei R, Mistretta A, Marventano S, Calabrese G, Masuelli L, Giganti MG, Modesti A, Galvano F, Gazzolo D. Effects of vitamin C on health: a review of evidence. Frontiers in bioscience (Landmark edition). 2013 Jun 1:18(3):1017-29 [PubMed PMID: 23747864]