Introduction
Bronchial carcinoid tumors (BCTs) are rare neuroendocrine tumors, potentially secreting biologically active chemical substances. They account for 1% to 2% of all lung tumors. BCTs were formerly known as bronchial adenomas, however, this terminology is no longer used as they are not glandular nor are they consistently benign. They are typically a slow-growing neoplasm with varying behavior, however, they can grow rapidly and overall be much more aggressive.[1]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
BCTs are most commonly endobronchial lesions however, up to 20% can be present as peripheral pulmonary nodules. They constitute between 1% and 5% of all lung cancers and are usually sporadically occurring malignancies.
Epidemiology
The mean patient age at diagnosis of bronchial carcinoid tumors is 45 years. It affects both males and females equally. Patients who are diagnosed with typical carcinoids are approximately one decade younger than those diagnosed with atypical carcinoids. 15% of cases metastasize, usually to the liver, bone adrenal glands, or brain. There is evidence that the prevalence is gradually increasing, which may be at least in part due to advanced diagnostic techniques.
Risk Factors
BCTs are known to be sporadically occurring, although there are instances associated with multiple endocrine neoplasia type 1 (MEN-1) syndrome. There are no environmental factors, such as smoking or inhaling carcinogens, that increase the risk of developing BCTs.[2]
Pathophysiology
BCTs develop from Kulchitsky cells found within the bronchial mucosa. They also develop from tracheobronchial tree cartilage, more specifically, the epithelium of this cartilage. The difference between carcinoid tumors and tumorlets is that BCTs exhibit neuroendocrine function. They secrete substances, which are typically peptide hormones and neuroamines, which secrete serotonin, ACTH, somatostatin, and bradykinin.
Bronchial carcinoid tumors are further subclassified into typical and atypical carcinoid tumors based on the presence or absence of tumor suppression marker mutations. Typical BCTs lack mutations or abnormalities of the p53, BCL2, or BAX genes, while atypical tumors have known expression abnormalities of these genes.
The majority of BCTs arise from the hilum or perihilar region of the lung, 75% of which arise in the lobar bronchi. In comparison, 10% and 15% arise from the mainstem bronchi and periphery, respectively. These tumors are highly vascularized and polypoid masses, which are less than 3 to 4 cm in size.[3]
Histopathology
Histologically carcinoid tumors of the lung are similar to those found in the intestine. They appear as nests and cords of small, round, uniform cells. Atypical carcinoids differ in that they tend to be more aggressive, as evidence by an increased number of mitoses and more pleomorphism. Atypical BCTs have a greater tendency to invade nearby lymphatics. Statistics show that 90% of BCTs are well-differentiated compared to 10% of atypical cases, which have more pleomorphism.[4]
History and Physical
High suspicion of diagnosis is needed to identify bronchial carcinoid tumors, which often leads to delayed diagnosis. 35% of patients will present with either productive or nonproductive cough. Others may present with wheezing, which is often misdiagnosed as asthma and has a prolonged treatment course without improvement. Approximately 25 to 50% of patients will report hemoptysis, while 40% will report a history of chest pain or evidence of bronchial obstruction.
Other presentations could be consistent with recurring pneumonia distal to the obstruction caused by the tumor. Rarely will patients experience carcinoid syndrome while the tumor is located in the tracheobronchial tree. If a patient has neuroendocrine production of ACTH due to the carcinoid tumor, they may present with Cushing's syndrome. A large portion of bronchial carcinoids, up to 25%, are asymptomatic cases.
Evaluation
75% of cases are detected on a chest X-ray. Often incidental findings of pulmonary nodules were found on x-ray. As BCTs are commonly central lesions of the tracheobronchial tree, findings related to bronchial obstruction are commonly found. Overinflation of the lung as a result of air trapping due to the lesion can be seen as well as peripheral atelectasis distal to the lesion if the occlusion is large enough. 40% of cases will have atelectasis, while 25% will show a central lesion on Chest X-ray. Homogenous increased opacities with volume loss may also be found on radiographs. In roughly 20 % of cases, BCTs develop in the periphery of the lung identified as a peripheral solitary nodule.
Further investigation with computed tomography of the thorax can help identify smaller lesions of the tracheobronchial tree. Common findings include contrast enhancement of the highly vascularized lesion and/or calcification. A new CT sign called "bronchial tree sign," as explained by Dr. Coruh et al. can be used to differentiate Ct findings of carcinoid tumors from hamartomas.[5][6][7] Rarely will carcinoids be found in mediastinal structures, and multifocal tumors are almost never seen.
Given the high likelihood of central lesions, bronchoscopy is often the most effective tool in diagnosing bronchial carcinoid tumors. In conjunction, the measurement of biochemical markers in the body, such as urinary 5-hydroxyindoleacetic acid and serum chromogranin A can result in a more confident diagnosis.
To effectively differentiate typical and atypical BCTs, surgical specimens are preferred when compared to biopsy and cytology via the use of bronchoscopy. Differentiation is confirmed via immunostaining for neuron-specific enolase, serotonin, calcitonin, and bombesin. Mediastinoscopy is suggested only when there is moderate to high suspicion for atypical carcinoid tumors. Further imaging for metastases is also indicated should there arise high suspicion for atypical carcinoid given its more aggressive nature.
Treatment / Management
Complete surgical resection is the treatment of choice for bronchial carcinoids and usually confers a good prognosis, particularly in typical carcinoids. Evidence has shown excellent survival rates of 5, 10, and 15 years of 92%, 88%, and 76% respectively. Atypical carcinoids carry a worse prognosis with a 5 and 10-year survival rate of 69% and 24 to 52%, respectively. Surgical interventions include radical resection via pneumonectomy, bilobectomy, lobectomy, and more conservative approaches such as segmentectomy, wedge resection, and sleeve bronchectomy. The majority of cases are localized, with resection leading to better outcomes.
Stage IV bronchial carcinoid tumors have a slightly different approach to management. Surgical resection alone is not sufficient for patients diagnosed with late-stage disease. These patients are candidates for interventional radiology interventions in attempts to reduce the size of the tumor. Somatostatin analogs are used in these procedures not only to decrease tumor mass, but they also have beneficial effects in the symptomatic management of neurosecretory effects of the disease.[8] The use of somatostatin analogs can stabilize tumor growth for several years while providing symptomatic relief.[9]
In patients with advanced disease, chemotherapeutic agents such as temozolomide and thalidomide, although still under clinical trials, have shown therapeutic benefits. Other clinically studied chemotherapy options include radioisotope-labeled peptide receptor therapy with 131-MIBG, 90 Y-DOTA-TOC, or 177 Lu-DOTA-TOC have also shown promising results in their respective clinical trials. Further clinical trials with tyrosine kinase antibodies and inhibitors of the mTOR mammalian receptors are still currently under trial.[10][11](B3)
Differential Diagnosis
Differential diagnosis of a lung lesion is vast. They can be separated by malignant vs. benign etiologies. Malignant differential diagnoses include adenocarcinoma of the lung, squamous cell carcinoma, and large and small cell carcinoma.
Benign lesions of the lung can also be differentiated by category. Infectious granulomatous lesions such as histoplasmosis, coccidioidomycosis, tuberculosis, cryptococcosis, and blastomycosis. Benign neoplasms such as bronchial hamartomas, lipomas, fibroma, neurofibroma, leiomyoma, and angiomas. Vascular etiologies such as arteriovenous malformation, pulmonary varix, hematoma, and pulmonary infarct. Inflammatory origins such as granulomatosis with polyangiitis, rheumatoid nodule, or sarcoidosis. Other etiologies such as amyloidoma, round atelectasis, intrapulmonary lymph nodes, loculated fluid, and mucoid impaction should all be included in the differential.
Disorders with similar clinical manifestations of bronchial carcinoid tumors include obstructing bronchial carcinoma, endobronchial metastasis, asthma/reactive airway disease, as well as an aspirated foreign body.[12]
Surgical Oncology
Newly published studies, such as those reviewing 98 cases from 2014 to 2018 who underwent surgical resection, reported complete resolution of neuroendocrine-specific symptoms. This includes the disappearance of neuroendocrine markers once the tumor has been surgically removed.[13] In contrast to the aggressive surgical resection, further retrospective reviews have been performed evaluating the role of total lung-sparing surgical procedures. Among the 98 patients who underwent lung-sparing surgical resection, 38% were typical, and 10% were atypical carcinoids. The mean length of resected bronchus was only 2.2cm, with no perioperative mortality. The 5-year survival rate for lung-sparing surgical resection was 97% for these patients undergoing less aggressive surgical intervention.[14]
Pertinent Studies and Ongoing Trials
Very few clinical trials have been performed in regard to the medical management of pulmonary, endocrine tumors because of its rarity. However, there are ongoing randomized control trials such as Alliance A021602: CABINET. This trial is currently comparing the effects of cabozantinib vs. placebo for those patients with well-differentiated bronchial carcinoids. Other ongoing trials include an open-label phase II trial that is currently assessing the use of ipilimumab and nivolumab in advanced disease. The data from these studies are still being collected with anticipated results available in the near future. Ongoing studies such as these may highlight a much larger role of medical management as compared to surgical resection.[9]
Staging
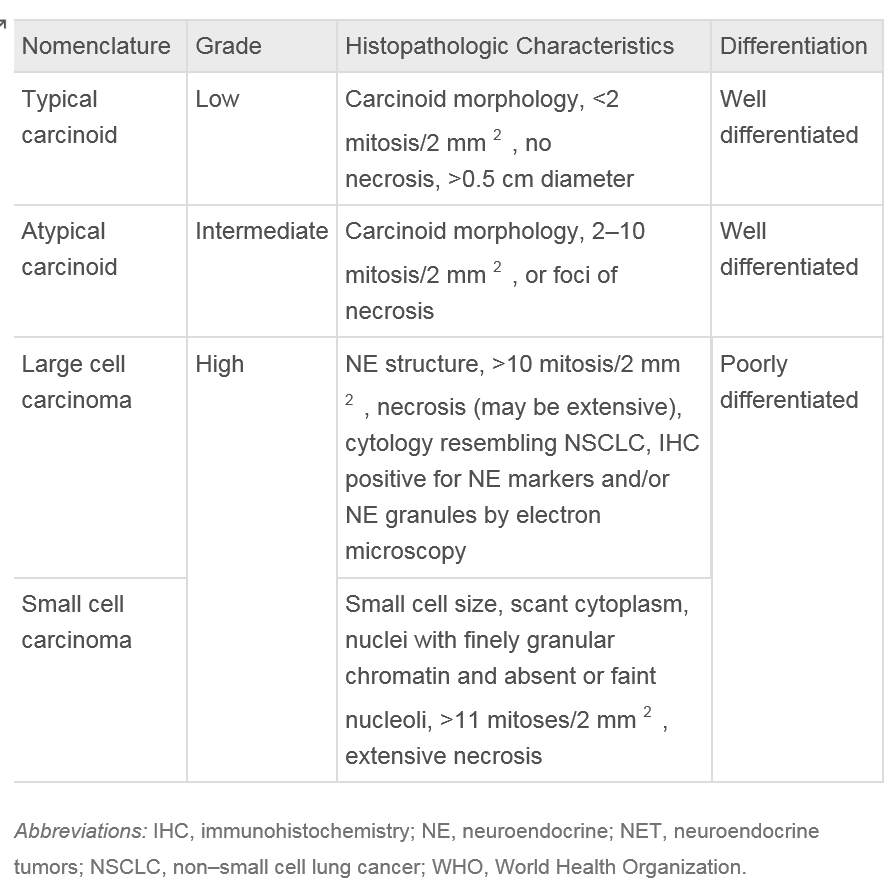
Classification of bronchial carcinoid tumors is dependent on the observation of mitosis found within the histology of the specimen. Typical carcinoids lack abnormalities in the expression of common tumor suppressor genes and are defined as observing less than 2 mitoses under microscopy of a 2 mm portion of a viable tumor. In contrast, atypical carcinoids not only have known mutations or abnormalities in gene expression but consist of 2-10 mitoses, necrosis, or pleomorphism of the biopsied sample. Since changing the guidelines for typical carcinoid tumor classification in 1998, the 10-year survival rate of confirmed typical carcinoid tumors has increased to 87% compared to 73% previously observed [13]. TNM staging system and mitosis criteria again updated by the World Health Organization in 2015 are listed below in table 1.
The common TNM staging classification is used for BCTs as it is used for various other lung cancers. Primary carcinoids of the pulmonary symptoms account from 0.4-3% of all resected tumors of the lung and roughly 25% of all carcinoid tumors. All carcinoid tumors of the lung have the potential to invade local structures and metastasize and thus are classified as malignant lesions. Although other cancers of the lung, such as small cell lung cancer, can have neuroendocrine granules, there is no relationship between these and bronchial carcinoid tumors as based on genetic analysis of these separate lesions.
Bronchial carcinoid tumors are classified under the World Health Organization (WHO) criteria, updated in 2015. These tumors range from typical carcinoids (TC), which are considered low grade, to high-grade tumors, which are very aggressive.
Prognosis
Prognosis of BCTs depends upon a variety of factors, including intrabronchial obstruction, ability to metastasize, and overall production of vasoactive amines. The 10-year survival rate of typical carcinoid tumors of the lung is as high as 87-97% in some studies. In contrast, atypical carcinoid tumors of the lung carry a poorer prognosis with a 5-year survival rate of 56% and a 10-year survival rate of 35%.[15]
Important to note in prognosis is that those who underwent resection of typical carcinoid tumors have a much higher 10-year survival rate. Those who underwent surgical complete surgical resection have a significantly better prognosis. Recurrence is less common with typical carcinoids and is more likely with atypical carcinoid tumors contributing to the poorer prognosis, as mentioned previously above.
Complications
Although rare, at the time of biopsy or surgical resection of the lesion, the patient potentially can undergo a carcinoid crisis due to excess secretion of neurosecretory granules. This is combated with the use of somatostatin analogs. Carcinoid syndrome is a rare complication and not common with primary bronchial lesions without metastasis. Cushing’s syndrome may result as a result of excess ACTH production from neurosecretory lesions.[15] Rarely bronchial carcinoid tumors metastasize; however, there have been reported cases of suprasellar lesions as a result of metastasis.[16]
Deterrence and Patient Education
Education involving the patient, healthcare provider, and the general public in order to provide proper education and disease awareness to better patient outcomes.
Enhancing Healthcare Team Outcomes
Bronchial carcinoid tumors are a recurring issue in the medical field that is often overlooked due to its relatively nonspecific initial findings. It is greatly important that the pathophysiology of the disease is understood, and suspicions heightened for such a disease. Although surgical resection is the treatment of choice, it has already been proven that medical therapy has had a significant role in the disease course. Symptomatic relief and deterrence of disease progression will undoubtedly lead to improved patient outcomes, in particular to those patients who achieve earlier diagnosis. It is the ethical responsibility of the physician attending to the patient to provide the least invasive therapy when establishing treatment prior to recommending surgical resection. With an interprofessional approach throughout the disciplines of medicine, radiology, surgery, oncology, and pulmonology, there is no doubt that overall outcomes will continue to improve in the future.
Media
(Click Image to Enlarge)
References
Litvak A,Pietanza MC, Bronchial and Thymic Carcinoid Tumors. Hematology/oncology clinics of North America. 2016 Feb; [PubMed PMID: 26614370]
Davila DG,Dunn WF,Tazelaar HD,Pairolero PC, Bronchial carcinoid tumors. Mayo Clinic proceedings. 1993 Aug; [PubMed PMID: 8331983]
Piro R,Tonelli R,Taddei S,Marchioni A,Musci G,Clini E,Facciolongo N, Atypical diagnosis for typical lung carcinoid. BMC pulmonary medicine. 2019 Sep 2; [PubMed PMID: 31477066]
Pregun I,Bodoky G,Rácz K,Tulassay Z, [Carcinoid tumors]. Orvosi hetilap. 2010 Nov 14; [PubMed PMID: 21044939]
Tscherne G, [Sources of error in the diagnosis of trophoblastic tumors]. Wiener medizinische Wochenschrift (1946). 1977 Sep 30 [PubMed PMID: 200020]
Coruh AG,Kul M,Kuru Öz D,Yenigün B,Cansız Ersöz C,Özalp Ateş F,Atasoy Ç, Is it possible to discriminate pulmonary carcinoids from hamartomas based on CT features? Clinical imaging. 2020 Jun [PubMed PMID: 32066033]
Danti G,Berti V,Abenavoli E,Briganti V,Linguanti F,Mungai F,Pradella S,Miele V, Diagnostic imaging of typical lung carcinoids: relationship between MDCT, {sup}111{/sup}In-Octreoscan and {sup}18{/sup}F-FDG-PET imaging features with Ki-67 index. La Radiologia medica. 2020 Aug [PubMed PMID: 32189174]
Detterbeck FC, Management of carcinoid tumors. The Annals of thoracic surgery. 2010 Mar; [PubMed PMID: 20172187]
[Development of gustatory buds and its nervous fibers in the human tongue]., Brzezińska B,, Czasopismo stomatologiczne, 1977 Nov [PubMed PMID: 32862320]
Porziella V,Cesario A,Lococo F,Cafarotti S,Margaritora S,D'Errico G,Granone P, The radioguided 111In-pentetreotide surgery in the management of ACTH-secreting bronchial carcinoid. European review for medical and pharmacological sciences. 2011 Jun; [PubMed PMID: 21796863]
Level 3 (low-level) evidenceO'Byrne KJ,Halmos G,Pinski J,Groot K,Szepeshazi K,Schally AV,Carney DN, Somatostatin receptor expression in lung cancer. European journal of cancer (Oxford, England : 1990). 1994; [PubMed PMID: 7833144]
Bora MK,Vithiavathi S, Primary bronchial carcinoid: A rare differential diagnosis of pulmonary koch in young adult patient. Lung India : official organ of Indian Chest Society. 2012 Jan; [PubMed PMID: 22345917]
[Use of in vitro tissue culture in stomatological studies]., Kralisz W,, Czasopismo stomatologiczne, 1977 Nov [PubMed PMID: 33123230]
[2 cases of odontogenic septicemia with atypical clinical course]., Plamieniak Z,Medraś M,Man W,, Czasopismo stomatologiczne, 1977 Nov [PubMed PMID: 33096073]
Level 3 (low-level) evidenceBouledrak K,Walter T,Souquet PJ,Lombard-Bohas C, [Metastatic bronchial carcinoid tumors]. Revue de pneumologie clinique. 2016 Feb; [PubMed PMID: 26831129]
Christopher S H,Kundishora AJ,Elsamadicy AA,Koo AB,Beckta JM,McGuone D,Erson-Omay EZ,Omay SB, Genetic characterization of a case of sellar metastasis from bronchial carcinoid neuroendocrine tumor. Surgical neurology international. 2020 [PubMed PMID: 33093980]
Level 3 (low-level) evidence