Burn Debridement, Grafting, and Reconstruction
Burn Debridement, Grafting, and Reconstruction
Introduction
In 2015 it was recorded that burn injuries caused 500000 burns victims and 40000 hospitalizations in the United States. Over $7.9 billion is estimated to be spent on emergency room visits and hospital burn care per year. Survival after burns is attributed to a better understanding of when to transfer to burn centers, resuscitation protocols, and early excision and grafting. Over the last 50 years, there have been significant developments in the assessment and treatment of burn patients attributable to interprofessional care and increasing knowledge in the critical care of the burn patient.[1] These advances in treatment contribute to the improved morbidity and mortality of patients with over 20% total body surface area (TBSA) burns that would have previously not survived or had poor outcomes. Evidence-based data has significantly increased in recent years and is now defining the best treatment decisions as more literature gets published.[2][3]
Issues of Concern
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Issues of Concern
Skin Anatomy and Burn Depth
The largest organ of the human body is the skin. It protects against trauma, radiation, microorganisms, and provides regulation of temperature and tactile sensation. The epidermis is the outermost layer of the skin and is avascular. This section is made up of keratinocytes, which create a protective barrier against the outside environment. The epidermis can be 10 to 100 cells thick, depending on the location on the body. The dermis is found beneath the epidermis and is the mechanical strength layer that also functions as the main metabolic and nutrient-dense layer due to the blood vessels that penetrate this layer from below. Sweat glands and hair follicles are present in this layer. The hypodermis is composed of a layer of fatty connective and areolar tissue as well as nerves and larger blood vessels that help supply the more superficial layers of the skin. All layers of the skin contain immune-competent cells that assist in the protective barrier created by the skin.[4][5]
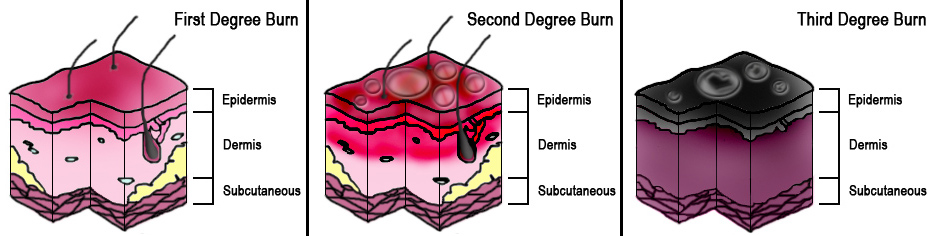
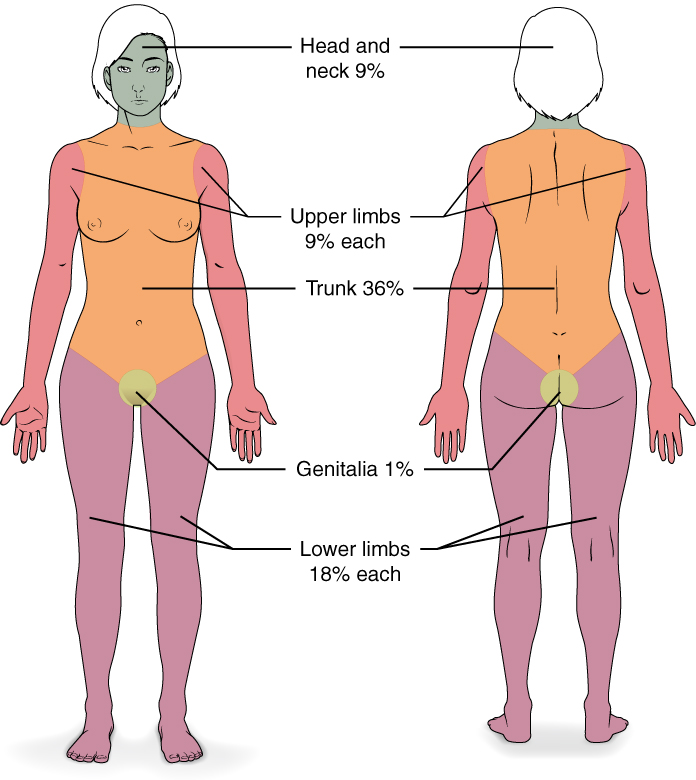
The total body surface area (TBSA) of the burn and burn depth are the best predictors of morbidity and mortality and determines the treatment steps. Superficial (first-degree) burns are defined as burns to the epidermis and usually cause minimal damage and no blisters. Erythema and pain are characteristic of superficial burns and include injuries such as sunburns or small flash burns. The affected epidermis sloughs off in 3 to 4 days as keratinocytes regenerate.
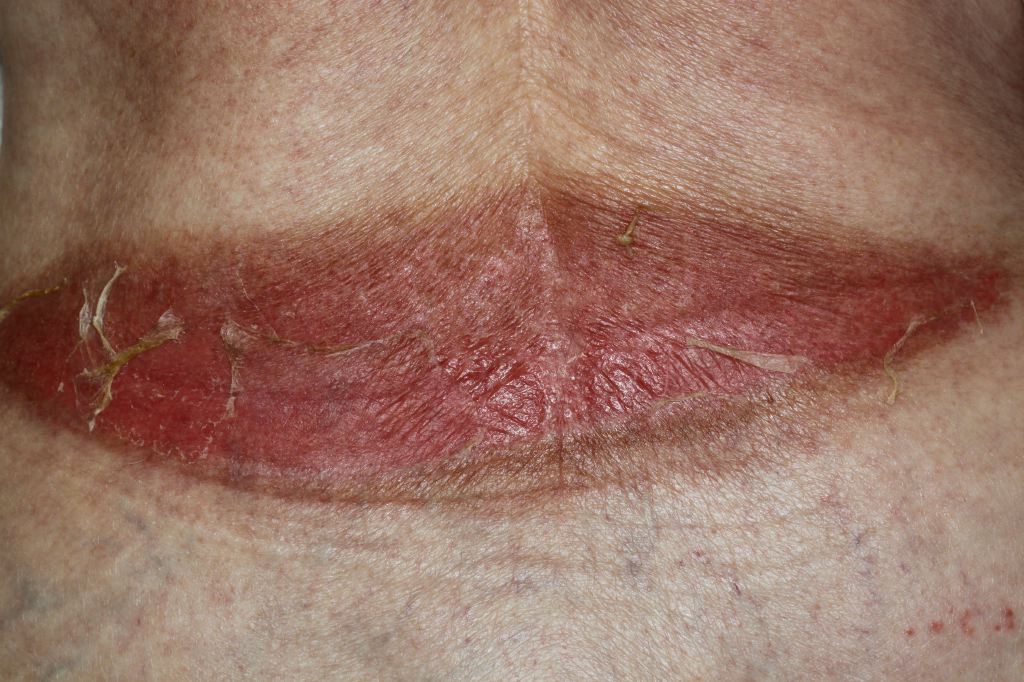
Burns that penetrate the dermis are partial-thickness burns (second-degree), and depending on the depth of penetration, partial-thickness burns further characterize as superficial partial-thickness or deep partial-thickness. These burns penetrate the dermo-epidermal junction and create blisters that cause more apparent damage. Once this layer has been burned and structurally damaged, the skin can only repair itself when the basal layer of keratinocytes regenerates the skin layers. The superficial partial-thickness burn extends to the papillary dermis and is more painful than a superficial burn, produces exudative fluid, and easily blanches. It can take 10-14 days to heal completely with little to no scar formation. Deep partial-thickness burns reach the reticular dermis and may affect the hair follicles, nails, sweat, and sebaceous glands. Deep partial-thickness burns present similarly to superficial partial-thickness burns but have little to no blanching and have diminished sensation. Healing time will be over three weeks, and lead to noticeable hypertrophic scarring.
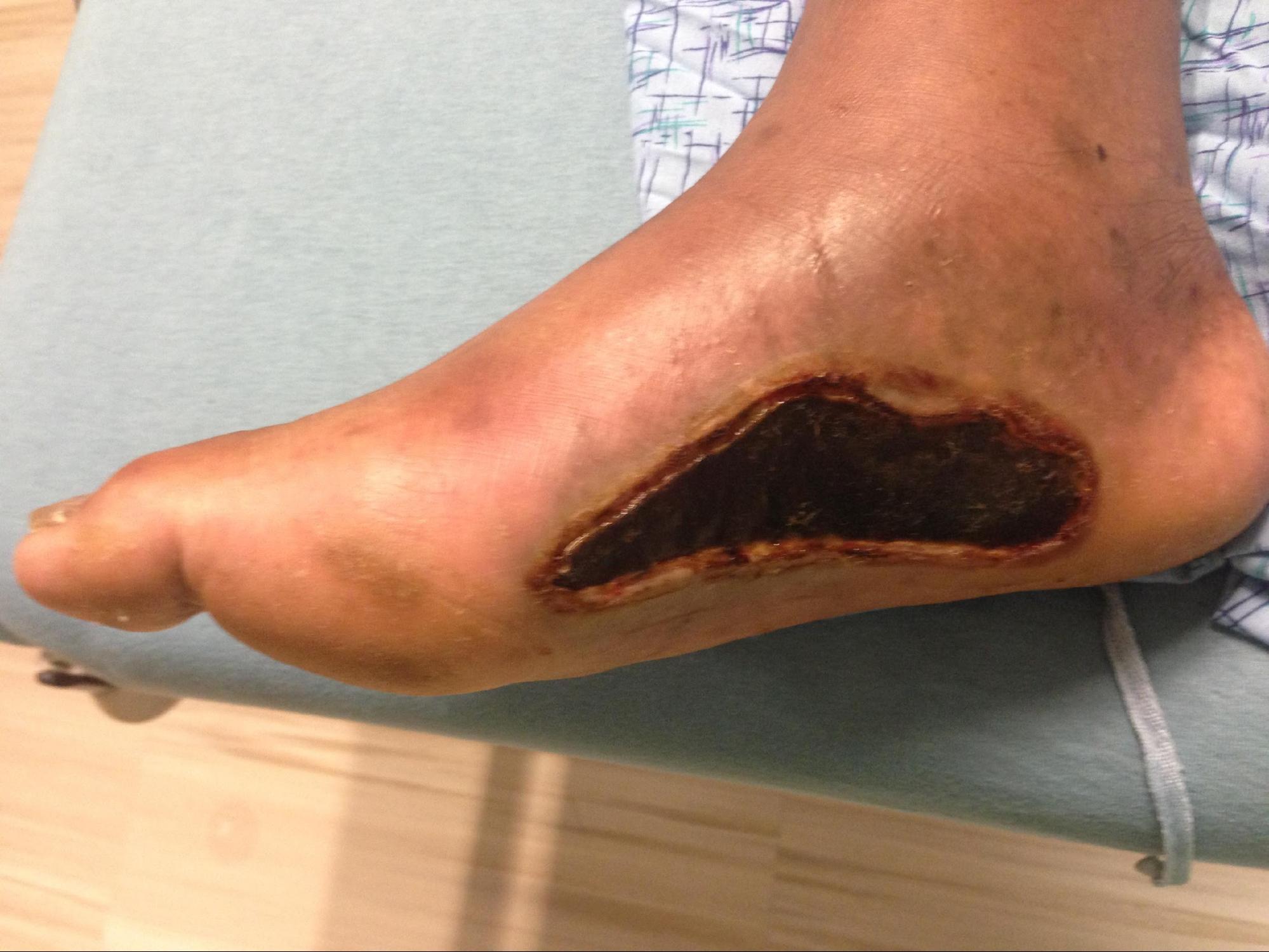
Full-thickness (third-degree) burns penetrate completely through the dermis and hypodermis, leaving a leathery and dry appearance to the skin. These burns are usually painless as the nerves that run in the subdermal plane are singed and destroyed along with the skin's blood supply. This layer is slow to heal and should be excised, which creates extensive scar formation that often requires reconstruction.[4][5]
Subdermal burns affect the layers beneath the cutaneous layer to include adipose tissue (fourth-degree), muscle (fifth-degree), or bone (sixth-degree). Surgical management and possible reconstruction are necessary for these burns.
The extent of the burn can divide into multiple regions. The zone of coagulation is where all cells are no longer viable and is at the central portion of the burn. Towards the periphery, a mixture of viable and necrotic cells are present, which is termed the zone of stasis. The viability of these cells is tenuous, and due to edema, infection, desiccation, or poor vascular flow from systemic hypotension, pressor use, and/or diabetes, these viable cells can become necrotic enlarging the zone of coagulation. On the periphery of the zone of stasis is the zone of hyperemia, where cells are viable, and there is vasodilation.[6]
Debridement
Upon presenting, the patient must undergo hydrotherapy or "shower" to have all devitalized tissue debrided, which is usually performed by the nursing staff. This process allows for the full extent of the burn to be visualized to determine an accurate %TBSA affected. When encountering a blister, it is best to un-roof it. If left intact, the blister becomes a potential source of infection, increases healing time, and negatively affects patient comfort and mobility. Additionally, blisters may hide deep partial-thickness burns underneath.[1] Of note, only second-degree burns and above are included in the calculation for %TBSA affected.
Early excision and grafting of the burn, noted as post-burn days 2 through 12, is the most important operation during the patients' hospital course. Early burn debridement is vital to the overall survivability and outcome of burn patients. Eschars and blisters need to be excised and/or opened as soon as possible to cease the inflammatory cascade causing secondary damage. The burned and dead tissue of partial-thickness and full-thickness burns create an opportunistic environment for gram-positive cellulitis to occur and leads to the sequelae of the wound burden, including slow healing time, physiologic impairment, contractures, and functional deficits. When burn patients undergo early excision and grafting, there is a decrease in the length of stay and faster healing time.[7][3][1] The delay of 24 to 48 hours before proceeding to the excision and grafting allows for the resuscitation and correction of physiologic derangements to optimize outcomes as well as providing time for the cells in the zone of stasis to become necrotic or demonstrate viability.
Logistical planning is necessary to determine what burns require excision and what areas are potentially useful as a graft donor site. The order of excision is dependent on the surgeon, but the goal is to safely excise and debride the largest surface areas first, such as the anterior or posterior trunk or large areas on the extremities. The size of the primary excision largely depends on the amount of available autograft or skin substitute, as it is essential to cover the excised and debrided areas. After removal of the burn eschar, the wound is open to the atmosphere, and the protective nature of the skin is compromised, which can allow for invasive infections and extensive fluid losses resulting in electrolyte disturbances and loss of oncotic pressure secondary to protein loss.[1] Generally, no more than 40% TBSA is excised at one time to prevent excessive blood loss and allow for complete coverage with autograft. For cosmetic areas and functional areas, excision should be performed at least within 7 to 14 days to improve outcomes.[8]
Surgical debridement is the gold standard of burn debridement and can be performed using a variety of tools. This process removes the necrotic tissue and foreign debris, which effectively decreases the bacterial load and allows for the excised skin to be cultured (if necessary). Surgical excision with the use of surgical knives such as the Weck/Goulian knife and the Blair knife has been the mainstay for surgical debridements. Large %TBSA and areas involving mixed partial- and full-thickness burns are best served by the use of the surgical knife technique for faster and more efficient debridement, which allows for precise and accurate preservation of healthy surrounding tissue allowing better wound healing and less scar formation.[8] Difficult areas to debride are contoured areas (e.g., inter-web spaces of the hands and feet), thin areas (face and dorsum of the hands), and edematous areas secondary to fluid resuscitation.[9]
One of the most critical aspects that affect the cosmetic outcome is dermal preservation.[7] Tangential excision of burned tissue involves unroofing the burn eschar and debriding the dead tissue layer by layer until encountering healthy bleeding tissue. This technique has improved overall morbidity and mortality because it allows for the removal of thin layers of burned tissue leaving viable tissue undisturbed.[7] This approach is opposed to a fascial excision, which is an excision of all skin elements to the subcutaneous fat/fascia. The depth of excision can be adjusted manually on the Blair knife, and preset guards are placed on the Weck/Goulian knife. Guards range from 0.004" to 0.028". The more common guards range from 0.008" to 0.012". Early tangential excision of partial- and full-thickness burns decreases healing time, reduces patient discomfort, prevents infection, and improves overall outcomes.
Surgical knives can sometimes result in incomplete or uneven debridement leading to multiple passes and prolonged operative time and increased bleeding.[7] Blood loss during surgical burn excision is a common problem, which can be in the range of liters lost. It is vital to have multiple units of packed red blood cells and plasma type and cross-matched preoperatively. Steps can be taken to avoid excessive blood loss using tourniquets, compressive dressings, limb elevation, infiltration, or topical application of epinephrine solutions, electrocautery, topical hemostatic agents (recombinant thrombin or fibrinogen), or staged excision and grafting. All those options have important uses in preventing anemia and hemodynamic instability secondary to blood loss intra-operatively.[1][5]
There is no consensus on what tools are best for the excision and debridement of wounds; this is left up to the preference of the operating surgeon and what resources are available at each respective facility. When deciding among the various techniques and tools for burn excision, consideration should be for the quality of life, physiology of the affected area, physical appearance, and overall survival.[7]
Additional debridement techniques are listed here:
- Hydrosurgical debridement (e.g., water jet and high-powered parallel water jet tools) is a new and useful tool in surgical debridement. Hydrosurgical debridement works by forcing a narrow stream of saline under high pressure out of a nozzle, using the Venturi effect, to remove the debrided tissue. It successfully clears bacteria from the wound bed, and this technique does not create bacterial seeding deeper into the wound bed.[10] Pressure settings can be adjusted to gently debride a superficial wound and remove pseudoeschar to debride deep partial-thickness burns aggressively. This process also effectively and precisely debrides fascia and tendon but does not affect bone or hard eschar. Eschars that are deep, hard, dry, or leathery are not easily debrided and require multiple passes as well as higher pressure settings.[8][9] Proponents of this technique advocate that this does not necessitate a big or intricate surgical instrument tray, which allows for quicker set-up and operating room turnover. The selectivity of hydrosurgical debridement saves money in the operating room. It decreases the number of operative take-backs for further debridement due to the specificity and efficiency of the debridements. The tool is small enough to debride hard-to-reach locations and obtain a better contour of the tissue to allow superficial to deep partial-thickness burns to be successfully excised more efficiently. The effectiveness of hydrosurgical debridement to produce healthy bleeding wound beds is comparable to cold knife and brush techniques but with better speed and precision. The advantages of hydrosurgical debridement are the ease in learning the technique and the reduction of total debridements, which lower operative costs. However, as previously stated, full-thickness burns will still require cold knife excision.
- Autolytic/Enzymatic debridement involves the use of proteolytic enzymes and agents that digest the burned and dead tissue. This process is limited in its use because it has a slower healing time and results in significant pain with dressing changes that require appropriate analgesia. The necrotic tissue is slowly debrided and can promote an invasive infection or even produce a septic inflammatory response.[8] Debridement with collagenase and papain/urea agents has shown success, but they are time and labor-intensive and not ideal in extensive TBSA burns.[8] Bromelain-based agents have some utility as they are highly specific for burn eschar. They do not penetrate the intact dermis and avoid the breakdown of healthy, viable tissue. Compared to surgical debridement, these agents have been found to decrease the total number of debridements, time to healing, and the need for autografting. Enzymatic debridement with bromelain-based agents allows for faster re-epithelialization and selective debridement down to bleeding, viable tissue. Another potential advantage for its use is the avoidance of escharotomy to avoid compartment syndrome caused by circumferential burns found in one study.[11] Financially, these topical debriding agents allow for a reduction in overall cost when used for burns less than 15% TBSA. At over 15% TBSA, the savings were no different than other types of debridement. Some of the disadvantages of using enzymatic debridement include an associated local redness to the treated area, which can be a cosmetic concern to some patients. There is also increased pain with the application and removal of the enzymatic agents when done bedside with no anesthesia.[7]
- Mechanical debridement can be utilized with frequent moist to dry dressing changes. This process involves placing a moist dressing over the affected area and then removing the dressing when it is dry. This technique removes the dead tissue adherent to the dressing but causes significant pain and can be nonspecific in removing surrounding healthy tissue.[8]
- Sterile maggots can be used in biologic debridement as they will only consume the necrotic wound tissue. This technique is a successful and effective strategy; however, there is hesitancy and apprehension from patients and healthcare staff when using this technique that limits real-world applications.[8]
Grafting
Early excision and grafting are directly related to improved survival rates. One study demonstrated a difference in mortality from 45% to 9% when full-thickness burns were excised and grafted within three days of injury compared to delayed grafting after 21 to 24 days.[12] However, for patients who are poor surgical candidates, surgery can be reserved for skin grafting alone, and surgical excision may be avoidable altogether. Once the burn sloughs off, the graft gets placed on healthy, viable granulation tissue. Prior to graft placement, bedside debridement with wound care must be thorough and pain well-controlled. Surgical risk merits consideration in all patients, and those with a delayed presentation, ongoing burn sepsis with aggressive fluid resuscitation, sepsis with or without multi-organ failure, and significant medical comorbidities are key factors in determining the timeline of excision and debridement.[1]
Two types of grafts are used to cover the wound bed; skin replacement and skin substitute. Skin replacement is the application of healthy skin onto the wound bed and includes autograft and allograft. Skin substitute is a mixture of cells or tissues that replaces skin autograft or allograft and is usually temporary or used in two-stage procedures. Skin substitutes include biomaterial and engineered tissue.[5]
Autograft is achieved by harvesting the patients' healthy skin and is the current standard of care in burn surgery. Autologous skin grafts can be full-thickness skin grafts (FTSGs) or split-thickness skin grafts (STSGs). The thicker the graft, the more primary contraction occurs (contraction immediately after harvesting) due to more dermal elements; however, there is less secondary contraction (contraction during healing). Thicker grafts, therefore, have a better cosmetic and functional outcome. Graft survival depends on the diffusion of nutrients and oxygen from the wound bed known as imbibition. Inosculation then follows when the blood vessels of the graft and from the wound bed grow together to make end-to-end contact. Lastly, neovascularization occurs when new blood vessels grow from the wound bed into the graft. Early failure of graft survival is attributable to seroma and hematoma formation, which lifts the graft off the wound bed, preventing imbibition. Other factors that lead to graft failure include shearing forces, edematous tissue, and infected tissue.
- STSGs consist of the epidermis and upper layer of the dermis and are the most commonly used for wound coverage. A dermatome is adjusted to a preset depth to harvest skin. Multiple brands of dermatomes are in production with their own unique depth settings, which can range up to 0.030", but typical STSG settings range between 0.010" to 0.012". When patients have extensive TBSA burns, STSG is a useful option because the same donor sites can be re-harvested after 1 to 2 weeks of healing. However, re-harvesting can cause the healed donor site to be hypopigmented, leading to hypopigmentation of any additional STSG taken from the same site. An option to cover more surface area is to cut slits in the skin graft, known as meshing the graft. Meshing the graft can increase the total surface area of the mesh by up to four times (settings being 1 to 1, 1.5 to 1, 2 to 1, 3 to 1, and 4 to 1), but can result in significant scarring with an irregular meshed pattern, contraction, and increased risk of infection.[13] Meshing has the additional benefit of preventing seroma and hematoma formation beneath the graft.
- FTSGs involve harvesting the epidermis and dermis completely to the underlying fat layer using a scalpel. This procedure is useful across joints or in cosmetic areas due to less scar and contracture formation. The major disadvantages of using FTSGs are the creation of a full-thickness wound at the donor site that needs to be closed primarily or require reconstructive flap closure. Additionally, FTSGs have a higher failure rate than STSGs due to a thicker layer of the dermis that must survive via imbibition.
Skin harvesting can result in a decent amount of blood loss. Steps can be taken to avoid excessive blood loss using compressive dressings, limb elevation, infiltration, or topical application of vasoconstrictors, electrocautery, and topical hemostatic agents. Injectable (tumescence) and topical epinephrine solutions are most commonly used to decrease the amount of bleeding and reduce transfusion requirements when excising large portions of donor skin.[1][5]
When the TBSA is larger than the available donor sites or tissues are too edematous to allow successful take of autograft, allograft and skin substitute is an alternative. The use of allograft has proven useful in burns with questionable readiness for autograft, complex burns, and burns of indeterminate depth.[3] An allograft is used as temporary coverage for up to 1 to 2 weeks, while edema is resolving and/or previous donor sites are healing and prepared for re-harvesting. Coverage of the excised burn is essential because leaving the wound bed open can create substantial fluid shifts, further injury, and significantly increase the risk of invasive infections. If the TBSA of the burn cannot be successfully covered after debridement, staged excision and grafting should occur so that all excised areas will have full coverage with some type of graft at the time of excision. The allograft is mostly obtained from human cadavers but is obtainable from living relatives who choose to donate; however, this is a rare occurrence and usually only used when treating pediatric burns. The allograft is cryopreserved and able to be tested to avoid transmission of disease. If the allograft remains on too long, the body will reject the tissue due to the antigens found in the donor epidermis associated with the Langerhans cells. However, the dermis of the allograft is inert and will not cause rejection. The dermal layer will integrate into the wound bed and promote wound healing, thus allowing delayed skin grafting with autograft to be successful.[1][5]
A xenograft is another option for temporary wound coverage. Porcine-derived products are most commonly used but put patients at risk for invasive infections if left in place for more than a few days. A xenograft is best used for temporary coverage of superficial partial-thickness burns and less so for deep partial-thickness and full-thickness burns due to infection risks.
Biosynthetic products are a mixture of bovine collagen, synthetic elements, human allograft, and porcine dermis. These dermal regeneration templates are acellular and create a scaffolding into which new skin can incorporate. These products are best used for major burns because they offer temporary wound coverage but require removal before skin grafting. Biosynthetic products are decent alternatives to autografting and allografting but are not definitive coverage options and carry a higher infection rate than autografting.
Amniotic membrane is a potential graft option used as a short-term dressing. It can is easily storable by techniques including irradiation, glyceral-preservation, or cryopreservation, which makes it easily accessible. However, it has limited use because of the expensive cost of virus testing.[1]
Cultured epithelial cells are a versatile graft used as an autograft or allograft. Thin slices of the epidermis can be harvested and cultured; however, this process is time-consuming, and burns should definitively receive grafting as soon as possible. Adherence and strength of the cultured epithelial cells on full-thickness burns are major disadvantages in its overall acceptance. Another disadvantage is that the cultured epithelial cells lack the dermal layer, which is a vital layer of the skin that promotes wound healing.[5] It is best used on top of the intact or regenerated dermis so that the cells can incorporate into the wound bed. Stem cells in suspension are not used commonly but have the potential for future use due to their broad application in previously meshed or grafted wounds, partial-thickness wounds, and wounds with the regenerated or intact dermis. [1]
Grafts are usually incorporated into the wound bed between postoperative days 3 to 5, which means that at this point, mobilization with an active range of motion and physical therapy can begin without fear of shearing the graft. Passive stretching and functional splinting should be performed as soon as possible to prevent graft contracture that may necessitate reconstruction.[1]
Reconstruction
Overall survival of the skin graft is important, but cosmetic outcomes and body image cannot be ignored when treating burn patients. Burn scars are a common occurrence after skin grafting and can cause anxiety, depression, pain, itching, altered pigmentation, temperature intolerance, and decreased range of motion secondary to scar contracture. Scar formation is propagated by deficiencies in the biosynthetic and tissue degradation pathway during wound healing. Fibroblast proliferation and excessive collagen production without degradation create hypertrophic scars and keloids. Scar contracture is a normal part of wound healing, but when the process gets altered, it can result in abnormal scar formation. Burn patients are hypermetabolic and in a pro-inflammatory state and subsequently experience prolonged wound-healing, which contributes to hypertrophic scar formation and alterations in pigmentation. The risk of hypertrophic scar formation increases if healing is not complete by 21 days. Surgical scar revision is the standard of care for burn patients who have contractures or poor cosmetic results. Surgical scar revision can be performed using skin substitutes, laser therapy, flaps, biomaterials, and other novel treatment approaches.[5]
Stem cells are potential treatments for fibrosis, contracture, and scarring but have yet to see an application in clinical practice. Native stem cells are involved in the normal healing process and create immunomodulation effects that promote overall wound healing, angiogenesis, and re-epithelialization. Stem cell therapy would involve surgical fat grafting into the wound site along with mesenchymal stem cells (MSCs) and adipose-derived stem cells (ADSCs). MSCs differentiate into various mesenchymal originating cells and decrease hypertrophic scarring by down-regulating collagen I synthesis and decreasing expression of myofibroblast markers. ADSCs decrease the incidence of hypertrophic scars and keloids by enhancing angiogenesis and decreasing inflammation to improve the size and overall cosmesis of the burn. Fat (adipose) grafting can potentially improve elasticity, wound healing, pain, color, range of motion, texture, softness, and thickness three months post-burn injury.[5][13]
Silicone is commonly used in skin substitutes to create a protective layer that acts as the new epidermis.[5] Silicone gel sheets, along with pressure dressings, have shown a dramatic decrease in pain, pruritis, and scar thickness six months after burn injury. One disadvantage of silicone is the limitation of placement. Silicone is difficult to keep in place at the joints and areas of the body with a high range of motion. However, silicone gel has been developed to overcome this limitation and allows for the application of a thin, transparent layer to the burn wound.[13]
Corticosteroid injection has been a mainstay in the treatment of hypertrophic scars and has been proven to reduce pain, pruritis, and prevent scar contractures. Corticosteroids work by decreasing collagen synthesis, increasing fibroblast and keratinocyte production, and speeding up collagen degradation. The drawback of using corticosteroids include hypopigmentation and subcutaneous atrophy. Additionally, there is no standard or suggested dose or course of treatment established.[13]
Botulinum toxin A is a neurotoxin derived from bacteria that inhibits acetylcholine release to inhibit muscle contraction. Injection of botulinum into the muscle underneath burns allows for relaxation of muscle and reduces wound tension, allowing the wound to heal and minimize scar formation.[13]
Laser therapy is an advantageous technique to improve cosmetic outcomes before and after burn injuries. When used before burn surgery, it allows the scars to be more pliable and improve the overall cosmesis of the wound. Three different lasers are in common use: wavelength-specific lasers and non-ablative or ablative fractional lasers. Wavelength-specific lasers work by ablating blood vessels leading to tissue hypoxia, which inhibits excessive collagen deposition and decreases fibroblast activity. Fractional lasers are used selectively and avoid surrounding healthy skin around the target area to stimulate tissue remodeling. Non-ablative fractional lasers are primarily useful for well-healed, atrophic, or flat scars. Ablative fractional lasers work similarly to the non-ablative fractional lasers but cause a stronger reaction of neocollagenesis and tissue remodeling. The fractional lasers create improved flexibility, a decrease in scar thickness, and overall improved function.[13]
Microsurgical flaps are a great surgical option utilized in the reconstruction of complex burn wounds. When used in deep burn wounds, flaps allow for one-stage reconstruction, thereby reducing operative procedures and optimizing cosmetic and functional outcomes. Patient selection is important when performing this procedure to assure flap survival. Patients must be hemodynamically stable with no compromise in blood flow for the flap to survive, which can severely limit patient selection.[14]
Skin tissue engineering is an evolving field of burn surgery with the end goal of creating a new and living tissue that fully replaces human skin. The engineering of skin tissues is difficult, and there must be good vascularization, specific-programmed migration of cells to stay within the wound, and appropriate tissue strength for biomaterials to improve wound healing and promote skin regeneration. Collagen and hyaluronic acid have been incorporated into sponges, films, gels, and matrices in many agents. Collagen promotes the adherence and growth of keratinocytes and fibroblasts, which promote wound healing. Hyaluronic acid is an essential component of the skin extracellular matrix that assists in wound repair. Elastin is involved in providing the scaffolding and cell mediating functions in human skin. Skin substitute products containing elastin allow for one-step procedures of skin grafting and improved skin graft incorporation. Two-stage procedures are commonly employed with the application of a skin substitute that must be incorporated into the wound over time, then followed by split-thickness skin grafting.[5][13]
Clinical Significance
Burns are a frequent reason for emergency room visits and will continue to be commonly presenting injuries. Understanding the basics of burn management is vital for the continued advancement of medical literature when it comes to burn care. Focus is essential on the process of burn treatment with an emphasis on early excision and grafting, followed by reconstruction if necessary. The care of burn patients is interprofessional, with decisions being multi-factorial. With appropriate education, healthcare providers can work together to advance the field of burn surgery over time.
Enhancing Healthcare Team Outcomes
Arguably the most crucial aspect of burn care is the interprofessional team approach. Management of burns can be expensive, and the interprofessional team must be flexible and able to adapt. When assessing patients and their needs, the team should determine what resources are available in addition to the overall skills of the interprofessional team. The burn team includes, but is not limited to: surgeons, intensivists, anesthetists, nurses, physical and occupational therapists, respiratory therapists, social workers, pharmacists, dieticians, and researchers.
The surgeon usually provides oversight and overall management of the patient, including the timing of the various procedures. The surgeon should lead daily rounds and include most, if not all, interprofessional teams. Nursing staff, dieticians, and physical/occupational therapists must work hand in hand to progress the patient to a point where they can heal appropriately. Nursing will provide wound care, monitor the patient, and administer medication. Infection concerns should of necessity involve a board-certified infectious disease pharmacist to help enact appropriate antimicrobial therapy. Physical, occupational, and exercise therapy is an important component of the interprofessional team for splinting needs and the patients’ functional recovery to achieve a good quality of life. While researchers may not actively participate in patient care, they are essential in the collection and reporting of patient data and outcomes for the burn registry, which allows for continual quality assessment and process improvement.[1][15] All these various areas need to collaborate and coordinate their efforts as an interprofessional team to provide optimal burn care leading to better patient outcomes. [Level 5]
The burn intensive care unit (BICU) needs to specialize in the care of acute burn patients, focusing on strict infection control policies, temperature regulation, and hydrotherapy. Burn patients are at increased risk of acquiring infection due to the loss of skin and the innate immune protection it provides to the body. Strict infection control protocols should be enforced by all members of the healthcare team and include hand hygiene, use of personal protective gear, microbial surveillance, and antibiotic stewardship. Housekeepers are an important part of the interprofessional team that clinical staff should not overlook due to their importance in maintaining a clean BICU. Temperature regulation is important in the BICU as well as in the operating room. Care must be taken by all members of the burn team to recognize and maintain an appropriate temperature for the acute burn patient. Increased ambient temperatures within patients' BICU room and in the operating room up to temperatures ranging to over 100 degrees Fahrenheit is necessary to hypothermia and prevent an increase in the patient’s metabolic rate, which will worsen the hypermetabolic and hypercatabolic state. Hydrotherapy refers to a dedicated shower area or area where patients are washed thoroughly to remove any gross contaminants, debride wounds to display the full extent of the burn, and jump-start wound care.[15]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
ISBI Practice Guidelines Committee, Steering Subcommittee, Advisory Subcommittee. ISBI Practice Guidelines for Burn Care. Burns : journal of the International Society for Burn Injuries. 2016 Aug:42(5):953-1021. doi: 10.1016/j.burns.2016.05.013. Epub [PubMed PMID: 27542292]
Level 1 (high-level) evidenceKowal S, Kruger E, Bilir P, Holmes JH, Hickerson W, Foster K, Nystrom S, Sparks J, Iyer N, Bush K, Quick A. Cost-Effectiveness of the Use of Autologous Cell Harvesting Device Compared to Standard of Care for Treatment of Severe Burns in the United States. Advances in therapy. 2019 Jul:36(7):1715-1729. doi: 10.1007/s12325-019-00961-2. Epub 2019 May 7 [PubMed PMID: 31065995]
Level 3 (low-level) evidenceIsrael JS, Greenhalgh DG, Gibson AL. Variations in Burn Excision and Grafting: A Survey of the American Burn Association. Journal of burn care & research : official publication of the American Burn Association. 2017 Jan/Feb:38(1):e125-e132. doi: 10.1097/BCR.0000000000000475. Epub [PubMed PMID: 27893575]
Level 3 (low-level) evidenceMartin NA, Falder S. A review of the evidence for threshold of burn injury. Burns : journal of the International Society for Burn Injuries. 2017 Dec:43(8):1624-1639. doi: 10.1016/j.burns.2017.04.003. Epub 2017 May 20 [PubMed PMID: 28536038]
Stone Ii R, Natesan S, Kowalczewski CJ, Mangum LH, Clay NE, Clohessy RM, Carlsson AH, Tassin DH, Chan RK, Rizzo JA, Christy RJ. Advancements in Regenerative Strategies Through the Continuum of Burn Care. Frontiers in pharmacology. 2018:9():672. doi: 10.3389/fphar.2018.00672. Epub 2018 Jul 9 [PubMed PMID: 30038569]
JACKSON DM. [The diagnosis of the depth of burning]. The British journal of surgery. 1953 May:40(164):588-96 [PubMed PMID: 13059343]
Edmondson SJ, Ali Jumabhoy I, Murray A. Time to start putting down the knife: A systematic review of burns excision tools of randomised and non-randomised trials. Burns : journal of the International Society for Burn Injuries. 2018 Nov:44(7):1721-1737. doi: 10.1016/j.burns.2018.01.012. Epub 2018 Feb 16 [PubMed PMID: 29456099]
Level 2 (mid-level) evidenceGranick MS, Posnett J, Jacoby M, Noruthun S, Ganchi PA, Datiashvili RO. Efficacy and cost-effectiveness of a high-powered parallel waterjet for wound debridement. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2006 Jul-Aug:14(4):394-7 [PubMed PMID: 16939565]
Level 2 (mid-level) evidenceRennekampff HO, Schaller HE, Wisser D, Tenenhaus M. Debridement of burn wounds with a water jet surgical tool. Burns : journal of the International Society for Burn Injuries. 2006 Feb:32(1):64-9 [PubMed PMID: 16376483]
Skärlina EM, Wilmink JM, Fall N, Gorvy DA. Effectiveness of conventional and hydrosurgical debridement methods in reducing Staphylococcus aureus inoculation of equine muscle in vitro. Equine veterinary journal. 2015 Mar:47(2):218-22. doi: 10.1111/evj.12284. Epub 2014 May 26 [PubMed PMID: 24750338]
Level 3 (low-level) evidenceKrieger Y, Rosenberg L, Lapid O, Glesinger R, Bogdanov-Berezovsky A, Silberstein E, Sagi A, Judkins K. Escharotomy using an enzymatic debridement agent for treating experimental burn-induced compartment syndrome in an animal model. The Journal of trauma. 2005 Jun:58(6):1259-64 [PubMed PMID: 15995479]
Level 3 (low-level) evidenceHerndon DN, Barrow RE, Rutan RL, Rutan TC, Desai MH, Abston S. A comparison of conservative versus early excision. Therapies in severely burned patients. Annals of surgery. 1989 May:209(5):547-52; discussion 552-3 [PubMed PMID: 2650643]
Level 1 (high-level) evidenceWang Y, Beekman J, Hew J, Jackson S, Issler-Fisher AC, Parungao R, Lajevardi SS, Li Z, Maitz PKM. Burn injury: Challenges and advances in burn wound healing, infection, pain and scarring. Advanced drug delivery reviews. 2018 Jan 1:123():3-17. doi: 10.1016/j.addr.2017.09.018. Epub 2017 Sep 20 [PubMed PMID: 28941987]
Level 3 (low-level) evidencePessoa Vaz M, Brandão C, Meireles R, Brito IM, Ferreira B, Pinheiro S, Zenha H, Ramos S, Diogo C, Teles L, Cabral L, Lima J. The role of microsurgical flaps in primary burn reconstruction. Annals of burns and fire disasters. 2018 Sep 30:31(3):233-237 [PubMed PMID: 30863259]
Cancio LC, Lundy JB, Sheridan RL. Evolving changes in the management of burns and environmental injuries. The Surgical clinics of North America. 2012 Aug:92(4):959-86, ix. doi: 10.1016/j.suc.2012.06.002. Epub [PubMed PMID: 22850157]