Introduction
Breast cancer is the most common cancer diagnosed in women and the second most common cause of death from cancer among women worldwide.[1] The breasts are paired glands of variable size and density that lie superficial to the pectoralis major muscle. They contain milk-producing cells arranged in lobules; multiple lobules are aggregated into lobes with interspersed fat. Milk and other secretions are produced in acini and extruded through lactiferous ducts that exit at the nipple. Breasts are anchored to the underlying muscular fascia by Cooper ligaments, which support the breast.[2]
Breast cancer most commonly arises in the ductal epithelium (ie, ductal carcinoma) but can also develop in the breast lobules (ie, lobular carcinoma). Several risk factors for breast cancer have been well described. In Western countries, screening programs have succeeded in identifying most breast cancers through screening rather than due to symptoms. However, in much of the developing world, a breast mass or abnormal nipple discharge is often the presenting symptom.[3] Breast cancer is diagnosed through physical examination, breast imaging, and tissue biopsy. Treatment options include surgery, chemotherapy, radiation, hormonal therapy, and, more recently, immunotherapy. Factors such as histology, stage, tumor markers, and genetic abnormalities guide individualized treatment decisions.[1]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Breast Cancer Risk Factors
Identifying factors associated with an increased incidence of breast cancer development is important in general health screening for women. Risk factors for breast cancer include:[4][5] (see Image. Breast Cancer Risk Factors)
Age: The age-adjusted incidence of breast cancer continues to increase with the advancing age of the female population.
Gender: Most breast cancers occur in women.
Personal history: A history of cancer in one breast increases the likelihood of a second primary cancer in the contralateral breast.
Histologic: Histologic abnormalities diagnosed by breast biopsy constitute an essential category of breast cancer risk factors. These abnormalities include lobular carcinoma in situ (LCIS) and proliferative changes with atypia.
Family history and genetic mutations: First-degree relatives of patients with breast cancer have a 2-fold to 3-fold excess risk for the development of the disease. Genetic factors cause 5% to 10% of all breast cancer cases but may account for 25% of cases in women younger than 30 years. BRCA1 and BRCA2 are the most important genes responsible for increased breast cancer susceptibility.
Reproductive: Reproductive milestones that increase a woman’s lifetime estrogen exposure are thought to increase breast cancer risk. These include the onset of menarche before age 12, first live childbirth after age 30 years, nulliparity, and menopause after the age of 55.
Exogenous hormone use: Therapeutic or supplemental estrogen and progesterone are taken for various conditions, with the most common scenarios being contraception in premenopausal women and hormone replacement therapy in postmenopausal women.
Other: Radiation, environmental exposures, obesity, and excessive alcohol consumption are some other factors that are associated with an increased risk of breast cancer.
Epidemiology
Invasive breast cancer remains the most common cancer among women worldwide, accounting for approximately 11.7% of new cases in 2020.[6] In the US, 1 in 8 women and 1 in 1000 men will develop breast cancer during their lifetime.[7][8][9] The incidence rate of breast cancer increases with age, from 1.5 cases per 100,000 in women aged 20 to 24 to a peak of 421.3 cases per 100,000 in women aged 75 to 79; 95% of new cases occur in women aged 40 years or older. The median age of women at the time of breast cancer diagnosis is 61 years.
A rapid increase in the incidence of breast cancer was noted until 2000, after which the incidence began to decline. More significant decreases occur in women younger than 50 years. With early detection and significant advances in treatment, breast cancer death rates have decreased over the past 25 years in North America and parts of Europe. In the US, breast cancer-related mortality dropped by 43% from 1980 to 2020. However, in many African and Asian countries (eg, Uganda, South Korea, and India), breast cancer incidence and death rates continue to rise.[6] Even within the US, marked disparity exists in detection and survival rates based on socioeconomic status and race. Although the incidence is highest among non-Hispanic whites, the mortality rate is significantly higher among African Americans. According to the American Cancer Society (ACS), breast cancer rates among women from various racial and ethnic groups are as follows:[10]
- Non-Hispanic white: 128.1 in 100,000
- African American: 124.3 in 100,000
- Hispanic/Latina: 91.0 in 100,000
- American Indian/Alaska Native: 91.9 in 100,000
- Asian American/Pacific Islander: 88.3 in 100,000
Pathophysiology
Most breast cancer is sporadic (90%-95%), with only 5% to 10% of patients having an identifiable genetic mutation.[11] BRCA 1 and 2 are the most common associated genetic conditions. Invasive ductal and invasive lobular carcinoma are the most common pathologic forms of invasive breast cancer. Carcinogenesis occurs due to a complex interplay of genetic and environmental risk factors, hormonal influences, and patient-related factors. The pathogenesis, treatment, and prognosis are closely associated with the following molecular subtypes of breast cancer:
- Luminal A: Hormone receptor-positive, human epidermal growth factor receptor (HER)-2 negative
- Luminal B: Hormone receptor-positive, HER-2 positive
- Basal-like: Hormone receptor and HER-2 negative
- HER-enriched: HER-2 positive, hormone receptor-negative
Hormone receptor-positive tumors (ie, luminal A and B) tend to be less aggressive, with improved survival rates.[12] HER-2 enriched tumors are more aggressive, with a poor prognosis without targeted therapy. In the era of targeted anti-HER therapy (eg, trastuzumab), the paradigm has shifted.[13] Basal-like tumors are negative for the molecular markers and tend to have a worse prognosis with poor survival rates.[14]
Histopathology
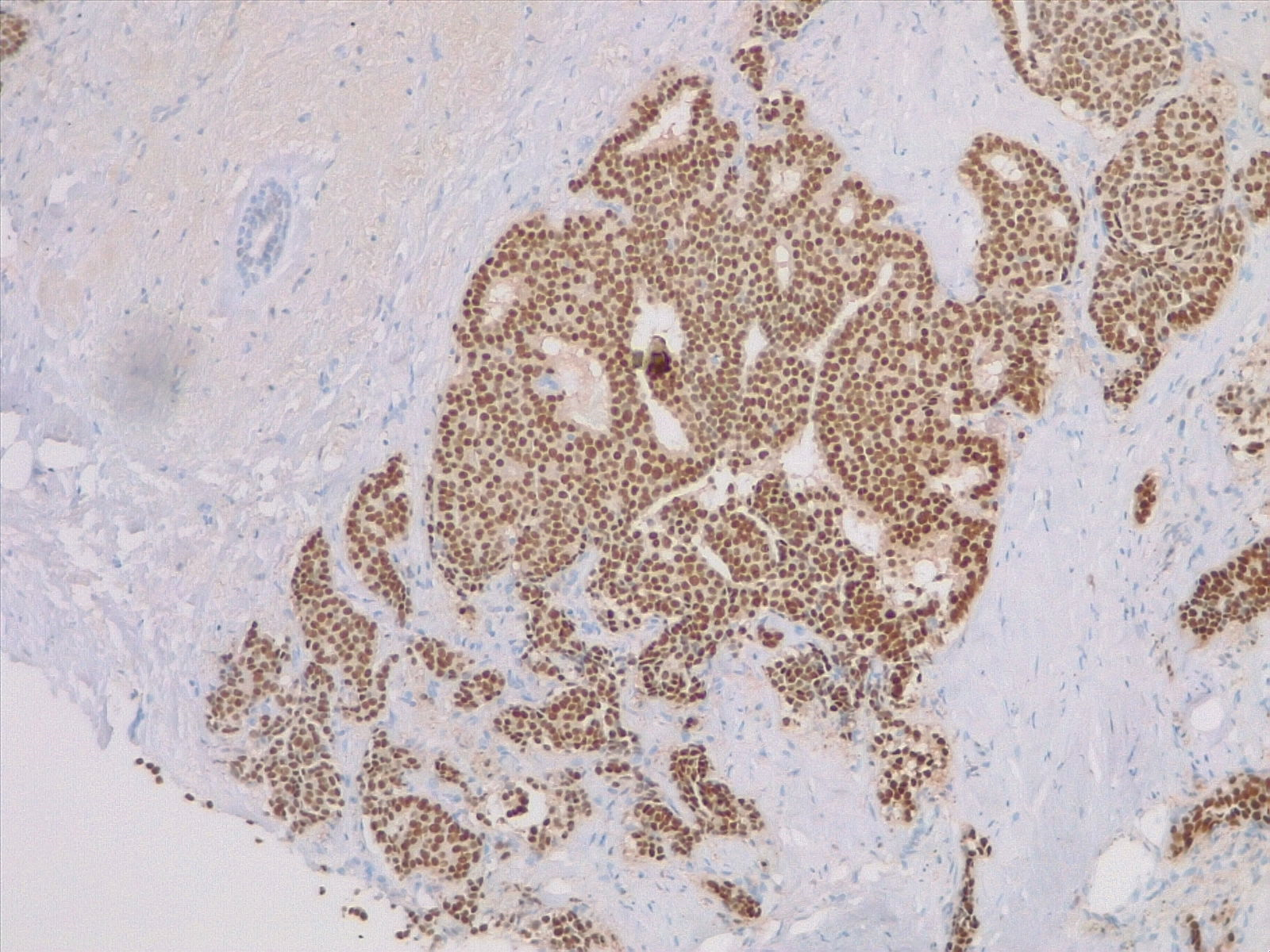
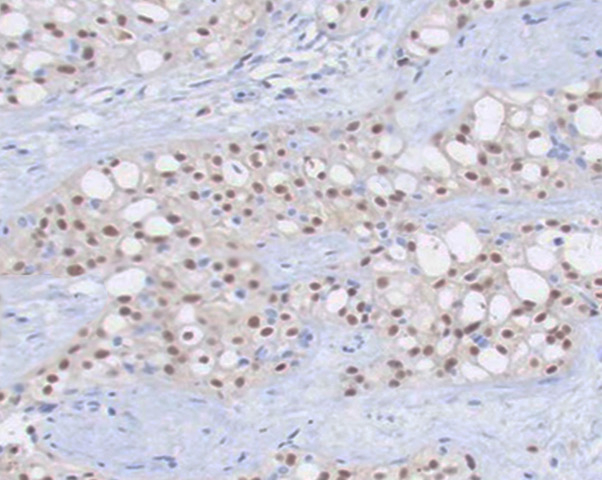
Invasive breast cancer is characterized by the invasion of neoplastic cells beyond the basement membrane that can be morphologically varied, with several subtypes described. All specimens should be tested for hormone receptors (ie, estrogen and progesterone) and HER-2 receptors. (see Image. Breast Estrogen Receptor Staining) Other critical components assessed on the histopathologic exam include tumor grade, pleiomorphism, Ki-67 index, morphology, tumor necrosis, multifocality, and precancerous lesions. The following are the most common histologic types of invasive breast cancer.
Ductal adenocarcinoma: This histologic type comprises 50% to 75% of all invasive breast cancers. Clinically, these tumors are often felt as a breast mass secondary to a significant fibrotic reaction. Microscopically, the lesion arises in the terminal duct-lobular unit with abnormal epithelial cells with varying degrees of atypia. These cells invade the basement membrane. However, there are no pathognomonic histologic features of invasive ductal carcinoma.[1] (see Image. Invasive Ductal Carcinoma).
Lobular carcinoma: Invasive lobular cancer makes up 10% to 15% of breast cancer and tends to permeate the breast in a single-file nature. This results in tumors that typically remain clinically occult, escaping detection on mammography or physical examination until the disease becomes extensive. A discrete mass is seldom palpated. Multifocal tumors and bilateral disease are more common with invasive lobular carcinoma. Characteristically, these tumors stain negative for E-cadherin.[15] (see Image. Pleomorphic Lobular Breast Carcinoma).
Mucinous carcinoma: Also known as colloid carcinomas, these tumors, which make up 2% to 5% of breast cancers, are well-demarcated in older women, typically characterized by mucin production.[16]
Tubular carcinoma: Microscopically characterized by infiltrating cells with minimal atypia that form small glands and tubules, 1% to 2% of breast cancers are among this subtype.[16]
Medullary carcinoma: These aggressive tumors are poorly differentiated and seen more commonly in BRCA mutant and younger patients.[17]
History and Physical
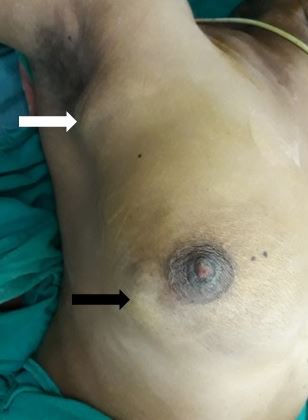
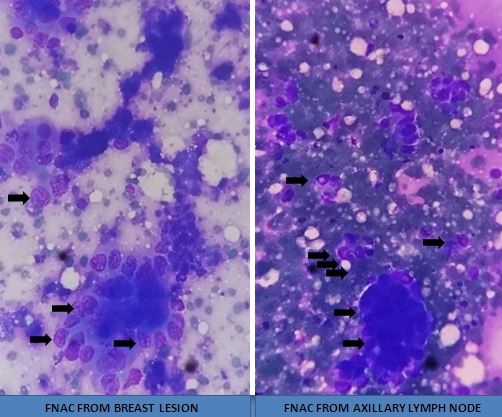
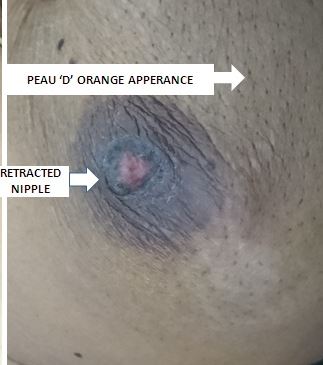
A periodic review of patient history for breast cancer risk assessment is recommended by the American College of Obstetricians and Gynecologists (ACOG).[18] Clinicians can use online assessment tools to help calculate a patient's breast cancer risk. Most breast cancer patients are asymptomatic, and lesions are discovered during routine breast examination or screening mammography. With increasing size, the patient may notice a palpable lump. Breast pain is an unusual symptom that happens 5% of the time.[19] More advanced disease may present with symptoms including peau d'orange, frank ulceration, axillary lymphadenopathy, or signs of distant metastasis. Inflammatory breast cancer, an advanced form of breast cancer, may have clinical features similar to breast abscess (eg, swelling, redness, and other local signs of inflammation).[20] (see Image. Breast Cancer Axillary Lymphadenopathy)
A thorough physical exam is a vital part of the clinical assessment for breast cancer. Both breasts must be examined in the sitting, standing, and supine positions, with the arm abducted, extended, and externally rotated. Palpation Overlying skin changes, nipple discharge, edema, peau d'orange, and ulceration should be noted. (see Image. Clinical Signs of Breast Carcinoma). Careful palpation of the regional lymph node basins for lymphadenopathy is also essential. Although some societies (eg, American Cancer Society) no longer recommend routine clinical breast examinations in asymptomatic, low-risk women as it has not been found to have a significant benefit, ACOG states that routine clinical breast examinations may be offered to these women, though not required. Furthermore, ACOG recommends an interval of every 1 to 3 years for women aged 25 to 39 years, and every year for women >40 years is appropriate if a screening breast examination is performed. However, a clinical breast examination should always be done for high-risk women and symptomatic women.[18] See StatPearls' companion topic, "Breast Examination Techniques," for additional information on clinical breast exams.[21]
Evaluation
Diagnostic Breast Imaging
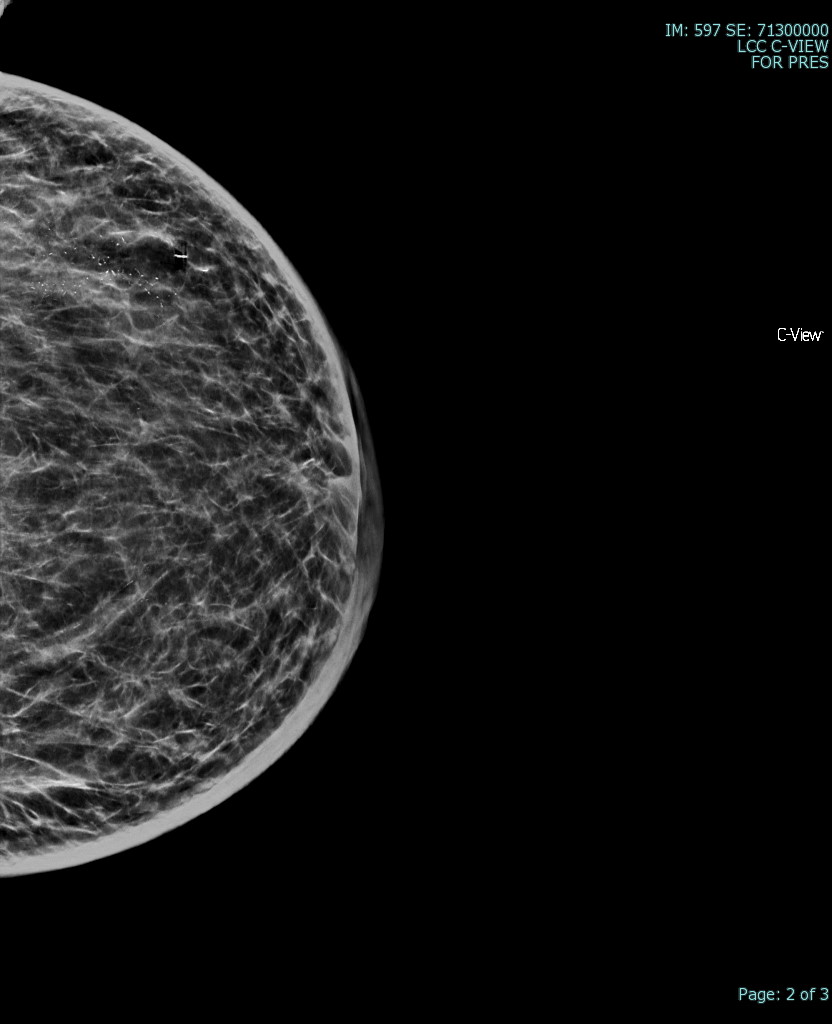
Mammography is the most commonly used modality for screening and diagnosis of breast cancer.[22] Abnormal findings on mammography include mass lesions, calcifications, or architectural distortion. When identified on screening mammography, diagnostic mammography, which utilizes higher quality imaging with several views, is indicated. Mammography is of limited utility in patients with dense breasts, in younger patients, and in those who cannot tolerate the breast compression that is required. Breast ultrasound or magnetic resonance imaging (MRI) with contrast may be utilized in such cases. Breast ultrasound is similar in sensitivity to mammography and can be used to obtain image-guided biopsy. Though MRI is the most sensitive imaging study, it is time-consuming, has limited availability, and is expensive.[23] Indications for MRI include axillary lymph node disease and an occult primary malignancy, Paget disease, multifocal or bilateral cancers, neoadjuvant chemotherapy treatment response assessment, and high-risk patient screening.[24] (see Image. Breast Mammogram)
Breast imaging findings are classified by their Breast Imaging Reporting and Data System (BI-RADS) category, which correlates imaging findings with their probability of underlying malignancy and recommends a broad treatment strategy. The BI-RADS categories range from 0 to 6.[25]
| Category | Findings | Recommendation | Cancer Probability |
| 0 | Inadequate | Repeat imaging | N/A |
| 1 | Negative | Continue routine screening | Close to 0% |
| 2 | Benign | Continue routine screening | Close to 0% |
| 3 | Probably benign | Follow up in 6 to 12 months | <2% |
| 4 | Suspicious (subdivided into 4a, 4b and 4c) | Biopsy | 2% to 95 % |
| 5 | Highly suggestive of malignancy | Biopsy | >95% |
| 6 | Biopsy proven | 100% |
Tissue Biopsy
Once a suspicious lesion is identified, tissue biopsy with stereotactic core needle biopsy is performed with imaging guidance.[26][27][28] Core needle biopsy is superior to fine needle aspiration and should be performed whenever possible.[29] In patients with clinically positive regional lymph nodes, an ultrasound-guided core needle biopsy is performed. Radiographically identifiable markers should be placed during the biopsy to mark the site in both the primary cancer and the lymph node basin to help identify and localize the lesion later. Breast tissue must be sent for a pathologic exam, including hormonal and Herceptin receptor testing.
Staging Imaging
Routine laboratory investigations and imaging for systemic disease are not recommended for operable breast cancer in the absence of symptoms. If associated symptoms are present, an MRI brain, chest CT scan, bone scan, or CT of the abdomen and pelvis may be performed as indicated. Baseline complete blood count and comprehensive metabolic panel, including liver function tests, are indicated if neoadjuvant chemotherapy is planned. For clinically advanced breast carcinoma (eg, inflammatory breast cancer, chest wall or skin involvement, and bulky axillary lymphadenopathy), a chest, abdomen, and pelvis CT along with a bone scan or an FDG-PET scan is often used.[30]
Treatment / Management
Breast cancer treatment is nuanced and based on various factors, including the disease stage, pathology, patient preference, and available resources. In general, breast cancer management approaches are divided into early breast cancer, locally advanced breast cancer, and metastatic breast cancer treatment.[30] (A1)
Early Breast Cancer
Early breast cancer includes tumors <5 cm in size without clinically positive lymph nodes. Treatment involves surgery, chemotherapy, radiation, and hormonal therapy, depending on the stage and molecular profile.[30] The modalities used include:(A1)
- Surgical treatment: Options to excise the primary tumor include breast conservation surgery (eg, partial mastectomy or lumpectomy) or a total mastectomy.
- Axillary lymph node management: Sentinel lymph node biopsy is performed during the operation. Without extranodal extension, no further axillary surgery is required if 2 to 3 axillary lymph nodes are microscopically positive. A completion axillary dissection or axillary radiation is indicated in patients with >3 positive lymph nodes or extranodal extension.
- Chemotherapy: Systemic chemotherapy is indicated based on the final stage and the tumor's molecular profile.
- In hormone receptor-positive tumors, the decision to initiate chemotherapy is based on risk stratification using genomic analysis of the primary using commercially available kits (eg, Oncotype Dx). High-risk patients benefit from chemotherapy in addition to hormonal therapy.
- All HER2-positive patients with tumors >1 cm should receive anti-HER2-directed therapy.
- All triple-negative patients with tumors > 1 cm should receive systemic chemotherapy.
- Radiation: Patients undergoing breast conservation surgery (BCS) must receive radiation to the breast with a boost to the tumor bed to reduce local recurrence. Patients who undergo mastectomy do not need breast radiation, except in certain circumstances (eg, >5 cm tumor, chest wall invasion, skin involvement, multifocal tumor, ≥4 positive nodes).
- Hormonal therapy: Anti-estrogen or aromatase inhibitor therapy is indicated in all hormone receptor-positive patients.
Up-front chemotherapy (ie, neoadjuvant therapy) has been increasingly used in early-stage triple-negative and HER2-positive tumors. Delivering the chemotherapy up-front has several advantages, including allowing response assessment, a greater likelihood of completing chemotherapy, and an increased likelihood of breast conservation therapy; therefore, clinicians will likely use this strategy more extensively.[31][32] (A1)
Locally Advanced Breast Cancer (LABC)
Locally advanced breast cancer (LABC) primarily consists of tumors larger than 5 cm or those with clinically positive lymph nodes. Most patients with LABC will receive some form of neoadjuvant therapy, with adjunct surgery and radiation therapy. Patients with LABC typically undergo a breast MRI at baseline. The primary tumor and the involved lymph nodes must have radiographically detectable markers placed before initiation of chemotherapy, as tumors can shrink and disappear after therapy.[30] (A1)
Chemotherapy regimens vary based on the tumor pathology (eg, hormone receptor-positive, HER2-positive, or triple-negative), the patient's age and physical status, and locally available resources. The goals of upfront chemotherapy are to reduce the size of the primary, eradicate micrometastatic disease, and assess disease biology based on the responsiveness of the tumor to chemotherapy. After completion of the chemotherapy regimen, breast and axillary imaging are repeated to assess response to chemotherapy and determine further management, including:
- Surgical treatment: Options to excise the primary tumor include BCS or a total mastectomy. Contraindications to BCS include large tumors, chest wall or skin involvement, multifocal disease, inability to receive radiation, and large tumor size to breast size ratio.
- Axillary lymph node management: In patients with a clinically positive axilla at diagnosis, an axillary dissection is always performed, regardless of the response of the tumor to neoadjuvant chemotherapy. In patients with a clinically negative axilla, sentinel lymph node biopsy is performed at the time of surgery. At least 3 lymph nodes should be harvested using a dual-tracer technique. Patients with residual disease should undergo a completion axillary dissection or axillary radiation.
- Systemic chemotherapy: Patients with residual disease after systemic chemotherapy may benefit from additional chemotherapy based on the molecular characteristics.
- Radiation therapy: The indications for radiation are similar to BCS.
- Hormonal therapy: Anti-estrogen or aromatase inhibitor therapy is indicated in all hormone receptor-positive patients.
Metastatic Breast Cancer
Metastatic breast cancer is managed primarily with systemic therapy. Chemotherapy, targeted therapy, immunotherapy, and hormonal therapy are all options, depending on the molecular profile and patient fitness. Palliative radiation may be used in controlling bulky primary disease and metastases to the brain, bone, and lung. Surgery is not recommended except for symptom control and palliative therapy.[33]
Differential Diagnosis
The differential diagnosis for breast cancer includes the following:
- Mastitis or breast abscess: Mastitis can be confused with inflammatory breast cancer. Inflammation or cellulitis that does not respond to antibiotics should be evaluated further.
- Fat necrosis: Traumatic fat necrosis can harden and present as a mass that mimics breast cancer.
- Fibroadenoma: Fibroadenomas >2 cm are typically excised to rule out coexisting breast cancer.
Surgical Oncology
Surgery plays a central role in managing breast cancer.[30] With the increased use of highly effective chemotherapy and targeted therapy, operations have become less extensive and morbid, while survival has improved. In current practice, surgery helps manage the primary tumor and provides essential staging information. BCS can be performed in most patients with tumors <5 cm, provided that the breast is large enough for a cosmetic result. Mastectomy is indicated in large primary tumors, tumors invading the skin or chest wall, multifocal cancers, inflammatory breast cancer, and in patients who are unable to have radiation. Sentinel lymph node biopsy is a vital staging procedure in patients with a clinically negative axilla. Those with 1 to 3 positive lymph nodes on sentinel node biopsy and without gross extranodal extension can safely avoid axillary lymph node dissection. Patients with clinically positive axillary nodes typically require an axillary lymph node dissection.[34] The following are the primary operations performed for breast cancer and in the axilla.
Partial Mastectomy or Lumpectomy
Partial mastectomy or lumpectomy involves the excision of a portion of the breast tissue with a margin of healthy tissue.[35] The incision can vary based on the location of the tumor and the desired cosmesis. Typically incisions are circumareolar, radial, or along the breast skin crease. Partial mastectomy is the centerpiece of BCS, allowing for the conservation of most of the breast. The cosmetic results depend on the amount of breast tissue removed compared to the remaining breast tissue and the nipple preservation. For nonpalpable lesions, the lesion must be localized preoperatively, usually with a wire or radioactive seed, to ensure the removal of the entire tumor.
Simple Mastectomy and Nipple-sparing Mastectomy
Simple mastectomy involves excision of the entire breast and nipple-areola complex.[34] The underlying pectoralis major fascia is removed as well. The amount of skin preserved can vary based on whether reconstruction is planned and on the type of reconstruction. A nipple-sparing mastectomy is a relatively recent modification of the simple mastectomy in which the nipple-areolar complex is spared, and the breast tissue is excised through a small circumareolar incision. The cosmetic results of reconstruction are superior to a conventional mastectomy, with a slightly increased but acceptably poorer oncologic outcome.
Modified Radical Mastectomy
Modified radical mastectomy combines the simple mastectomy technique with axillary lymph node dissection. The mastectomy incision is usually extended for the axillary contents to be removed. Radical mastectomy, which includes the removal of the pectoral muscles and sacrifice of the nerves, is seldom performed.
Axillary Sentinel Lymph Node Biopsy and Axillary Lymph Node Dissection
The axillary lymph nodes drain much of the ipsilateral breast and are divided into 3 levels by the pectoralis minor muscle. A radiotracer or blue dye is injected near the primary, and 1 to 3 lymph nodes in the axilla that have the highest uptake of radiotracer or are blue are excised. When done with a lumpectomy, the same incision can sometimes be used, or a separate incision at the axillary hairline may be required. Axillary lymph node dissection involves the removal of all the fibrofatty and lymphoid tissue in levels 2 and 3, with preservation of the long thoracic nerve and thoracodorsal nerve.[36][37]
Radiation Oncology
Radiation therapy has a significant role in local disease control, primarily in the adjuvant setting, but may also be used for palliative therapy. In early-stage breast cancer, adjuvant radiotherapy has been shown to reduce the risk of breast recurrent disease by approximately 50%.[38][39] While adjuvant radiotherapy in early-stage breast cancer has not been shown to improve overall survival, it is an essential part of the breast conservation approach as radiotherapy reduces the risk of recurrence and the need for additional surgery. Modalities to deliver adjuvant radiotherapy include external beam radiation, brachytherapy, or a combination.[40][41]
Radiation Therapy Delivery Techniques
Accelerated Partial Breast Irradiation
A select number of patients may qualify for Accelerated Partial Breast Irradiation (APBI). The American Society of Radiation Oncologists (ASTRO) appropriateness guidelines consist of suitable, cautionary, and unsuitable candidates for this treatment.[42] APBI may be delivered using surgically implantable single or multi-channel channel catheter devices. These implants rely on an Ir-192 HDR afterloader to deliver conformal radiotherapy via brachytherapy. (See StatPearls' companion topic, "Brachytherapy," for additional information.) Alternatively, APBI may be delivered using external beam radiotherapy. In this case, an implantable device is unnecessary, but surgical clips, coils, or 3D implantable markers may be used to delineate the surgical cavity for external beam radiotherapy planning. The dosing is 34 to 38.5 over 10 fractions delivered twice a day. The advantage of APBI is that it can be delivered over 1 week as opposed to 3 to 6 weeks with whole breast radiation. However, if the patient opts for APBI delivered via catheter, there may be additional delays as the patient would likely need to return for further surgery. In terms of outcomes, the 10-year cumulative incidence of breast cancer recurrence for patients treated with APBI was 4.6%.[43]
Whole Breast Radiation
Whole breast radiotherapy (WBRT) is a well-studied technique employed in patients with early-stage breast cancer and continues to be the mainstay treatment for many patients. WBRT is delivered in the adjuvant setting either after breast-conserving surgery or after the completion of chemotherapy. The treatment technique is designed to cover all visible breast tissue on CT simulation. This can be safely planned and delivered using a 3D conformal plan. The ipsilateral lung and heart doses are the most important to consider when planning these cases. Dosing varies from 40.05 to 50.4 Gy in 15 to 25 fractions. The 10-year ipsilateral breast recurrence rate in these patients is approximately 3.9%.[43]
An additional radiation dose, a boost, may be given to the surgical cavity upon completion of whole breast radiation. Several randomized trials have demonstrated an improvement with local control. Early-stage breast cancer patients who received a 10 Gy boost to the surgical cavity after whole breast radiation had a 5-year local recurrence rate of 3.6% compared to 4.5% without a boost. The EORTC demonstrated a 10-year local control rate of 6% versus 10% without a boost.[44] The benefit of a radiation boost appears to be confined to younger women aged <60 years.[44] The dosing ranges from 10 to 16 Gy. The boost is not without a cost, as there is a risk of breast fibrosis that may impact cosmesis. The EORTC trial found a 4.4% rate of severe fibrosis in patients receiving a boost compared to 1.6%.[44]
Post-Mastectomy Radiation
Post-mastectomy radiation (PMRT) is indicated in patients with nodal disease after axillary staging, positive margins, and in patients with primary breast tumors >5 cm. PMRT may also be considered in patients with high-risk pathologic features, including central or medial tumors ≥2 cm with either lymphovascular invasion, grade 3, or hormone receptor-negative. Coverage includes the chest wall with or without regional lymphatics. PMRT has been extensively studied in several prospective trials. The Danish 82bc trials investigated the benefit of PMRT in premenopausal and postmenopausal high-risk patients (ie, >5 cm, locally invasive, or node-positive). The study demonstrated long-term breast cancer mortality, locoregional recurrence, and overall survival benefits.[45] The 30-year follow-up data continues to show overall survival (19% versus 14%), breast cancer mortality (56% versus 67%), and locoregional recurrence (9% versus 37%) benefits.[45]
Comprehensive Nodal Irradiation
Comprehensive nodal radiation (CNI) covers all lymphatics draining the breast and chest wall, which consists of the levels I to III axilla, supraclavicular nodes, and internal mammary nodes. CNI can be incorporated into WBRT or PMRT and is indicated in node-positive patients, either from a sentinel node biopsy or axillary dissection. In patients undergoing an axillary dissection, the radiotherapy typically includes undissected areas and areas at risk for nodal involvement. CNI is technically more challenging than WBRT alone, requiring additional fields (ie, 3 or 4 field plans). CNI also increases the dose to uninvolved structures such as the lungs and heart. Meeting heart constraints may become especially challenging when treating the left breast. Certain techniques such as deep inspiratory breath hold (DIBH) or intensity-modulated radiation therapy (IMRT) may be helpful in these circumstances to minimize the amount of dose received by these structures. CNI has been prospectively compared to axillary dissections in patients with 1 to 3 nodes positive and was found to have similar rates of axillary control (0.93% versus 1.82%).[46] In addition, CNI has also been shown to improve 10-year disease-free survival (77% versus 82%) without an improvement in overall survival in high-risk patients.[47] Using CNI may also increase the risk of lymphedema as the regional lymphatics are radiated, making it more difficult to drain the breast and upper extremity. The additional dose to the lung may also increase the risk of radiation pneumonitis.
Intensity-Modulated Radiation Therapy
Breast intensity-modulated radiation therapy (IMRT) may be used as an alternative to conventional 3D planning in certain circumstances, such as failure to meet heart dose constraints, which is common, especially in patients with left-sided disease. Several prospective randomized trials have compared 3D or 2D planning to IMRT. They have consistently demonstrated that grade 2 or higher radiation dermatitis was significantly lower with IMRT than with 3D.[48][49] No differences in recurrence or survival were noted.
Radiation Therapy Complications
Cardiac toxicity
The risk of major coronary events as a long-term complication of breast irradiation has been well documented. Exposure of the coronary arteries may lead to accelerated atherosclerosis of the vessel, resulting in significant coronary events years after radiotherapy. A population case-control study demonstrated that the risk increases linearly with the dose to the heart, increasing the relative risk by 7.4% per gray without an apparent threshold.[50] Women with preexisting cardiac risk factors may have an even higher risk.[50]
Pneumonitis
The development of radiation pneumonitis in patients receiving adjuvant radiotherapy for breast cancer ranges from 0.8% to 2.9%.[51] Radiation pneumonitis has been documented in patients up to 1-year post-radiation and can require steroid treatment, oxygen therapy, and, in severe cases, intubation. The risk of pneumonitis increases with the volume of lung irradiated. Patients receiving comprehensive nodal RT are known to have higher rates of pneumonitis. The MA.20 study reported pneumonitis in 1.2% of their patients receiving regional nodal RT versus 0.2% in those treated to the breast only.[47] Concurrent use of taxanes such as paclitaxel, common in modern breast cancer chemotherapy regimens, may substantially increase the risk of pneumonitis in patients receiving radiation.[52] The most effective preventative measure is meticulous radiation planning and adherence to published lung dose constraints.
Breast fibrosis
Fibrotic changes in the breast are relatively common among patients receiving adjuvant radiotherapy. Onset is typically 4 to 12 months posttreatment, and the symptoms include breast shrinkage, hardening, pain, and poor wound healing. These changes can significantly affect cosmesis. The incidence in the literature ranges from 10% to 15%.[53] However, this risk of moderate to severe fibrosis may be influenced by several risk factors such as whole breast radiation dose, beam energy, dose heterogeneity, boost to the surgical cavity, and chemotherapy. A nomogram was developed using the data from the "Boost Versus No Boost" EORTC 22881-10882 trial to predict the risk of moderate to severe fibrosis in patients receiving whole breast radiation.[54] Preventative measures included weighing the risks and benefits of a breast boost, lowering beam energies, and limiting hot spots to <107% of the prescribed dose. In addition, patients at high risk for fibrosis may also take pentoxifylline with vitamin E for 6 months after radiation. This regimen has been shown in small randomized trials to reduce the risk of radiation fibrosis measured by a tissue compliance meter.[55] Unfortunately, once a patient has developed breast fibrosis, these changes are mostly irreversible. Management of patients with breast fibrosis consists mainly of symptomatic treatment, including NSAIDs, SNRIs, and anticonvulsants such as gabapentin.
Lymphedema
Progressive swelling of the upper extremity may occur in patients treated 6 months after radiation. The patient may notice increasing arm girth, swelling, heaviness, poor wound healing, and infection. The risk of developing lymphedema depends on the disruption to the regional lymphatics. The risk factors include the number of lymph nodes removed, body mass index, and amount of irradiated lymphatics.[56] A nomogram developed by Gross et al in 2019 may help quantify this risk.[56] Patients undergoing a sentinel node biopsy have a 5.6% risk of developing lymphedema compared with a 19.9% risk in those undergoing a full axillary dissection.[57] The AMAROS trial had a 5-year lymphedema rate of 25% in patients receiving an axillary dissection versus 12% in those receiving regional nodal radiation alone.[58] Patients receiving axillary dissection and regional nodal RT would be at the highest risk of developing lymphedema. Evidence for prevention is sparse but includes weight-bearing exercise and maintaining appropriate body weight. Patients with lymphedema may be managed with fitted compression garments, arm elevation, and exercise.
Brachial plexopathy
The brachial plexus trunks may be exposed to radiation doses in patients requiring regional nodal radiation. Symptoms include hand and arm paresthesia, weakness, and pain in the affected arm and shoulder. Onset is typically 8 to 12 months after treatment. Fortunately, this rare complication only affects approximately 1% of all patients. The risk may be increased in patients who have received chemotherapy or doses of radiation exceeding 50 Gy.[59] Primary prevention consists of limiting radiation doses to <50 Gy. Patients with brachial plexopathy may be managed with gabapentin and physical therapy.
Rib fracture
Rib fractures are another rare complication of breast radiotherapy, ranging from 0.3% to 1.8% of patients.[59][60] The median time to onset is approximately 12 months. The risk is associated with lower energies and higher doses of radiation. Treatment is generally conservative.
Secondary malignancy
Radiotherapy can induce DNA damage in both cancerous as well as normal tissues, which can lead to the development of radiation-induced malignancies years after treatment. Large meta-analyses have shown that patients receiving radiotherapy for breast cancer have an increased risk of non-breast cancers, including sarcomas, lung, and esophageal cancers.[61] However, the absolute risk of developing a secondary malignancy is low at 1% to 2% at 10 years.[62] Risk factors include age, gender, radiation field size, and radiation dose.[63]
Medical Oncology
Chemotherapy, hormone therapy, immunotherapy, and targeted therapy are the systemic therapies used in breast cancer management and are described below.
Cytotoxic Chemotherapy
Cytotoxic chemotherapy is used in the neoadjuvant and adjuvant setting. Chemotherapy is most effective in high-grade, poorly differentiated tumors that have a high cell turnover rate, such as triple-negative and HER2-positive tumors. The chemotherapy regimen depends on tumor characteristics, the patient's ability to tolerate chemotherapy, and the degree of potential benefit.[64]
Adjuvant chemotherapy is associated with improved overall survival, disease-free survival, and reduced local recurrence.[65] Cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) combination was one of the early regimens used in the adjuvant treatment of breast cancer. More modern regimens use anthracyclines (eg, doxorubicin or epirubicin) and taxanes in regimens such as TAC (ie, docetaxel, adriamycin, and cyclophosphamide). Adjuvant chemotherapy is recommended for most patients with triple-negative and HER2-positive tumors that are >T1 stage. Treatment recommendations for HR-positive tumors are more nuanced and are guided by commercially available genetic analysis kits (eg, Oncotype Dx, Mammaprint).[66][67] Neoadjuvant chemotherapy is increasingly used for triple-negative and HER2-positive tumors, which leads to increased compliance and tumor downstaging and allows assessment of the tumor's biological response.[68][69]
Targeted Therapy
- Anti-HER2 therapy is indicated in 17% of breast cancers that overproduce the growth-promoting protein HER2/neu. Trastuzumab, the first approved drug, is a monoclonal antibody directly targeting the HER2 protein. It reduces the risk of recurrence and death by 52% and 33%, respectively, if combined with chemotherapy in HER2-positive early breast cancer if compared to chemotherapy alone.[70][71] More recent data advocates for dual HER2 blockade with trastuzumab and pertuzumab, which improves response rates.
- PARP inhibitors (eg, olaparib and talazoparib) are monoclonal antibodies that prevent the activation of PARP, which are DNA repair enzymes. They are indicated in the adjuvant setting in individuals with BRCA mutations and HER2-negative breast cancer.[72]
- CDK4/6 inhibitors (palbociclib, target the CDK4/6 proteins, which promote cell division. Inhibition of this pathway promotes tumor lytic activity in HR-positive HER2-negative tumors. They are indicated in metastatic HR-positive, HER2-negative tumors and selected patients with early HR-positive tumors.[73]
- Immune checkpoint inhibitors (pembrolizumab, nivolumab) act on the PD-1, PD-L1 pathway to activate the host immune system. They are currently indicated in triple-negative breast cancer and the metastatic setting.[74]
Hormonal Treatment
Selective estrogen receptor modulators (eg, tamoxifen) or aromatase inhibitors (eg, exemestane and letrozole) are indicated in HR-positive breast cancers. Estrogen receptor modulators are especially indicated in premenopausal women, while both drugs can be used postmenopausal. Hormonal therapy reduces the risk of breast cancer recurrence and mortality and is indicated from 5 to 10 years.[69][31] Premenopausal women may also benefit from oophorectomy or chemical suppression of the ovaries (eg, GnRH antagonists), which are the primary source of estrogen before menopause.[75]
Staging
Breast cancer staging is determined clinically and histologically. Clinical breast cancer staging is based on physical examination and imaging studies before treatment. Histopathologic breast cancer staging is determined by pathologic examination of the primary tumor and regional lymph nodes after definitive surgical treatment. Staging is performed to group patients into risk categories that define prognosis and guide treatment recommendations for patients with a similar prognosis. Breast cancer is classified with the TNM classification system, which groups patients into 4 stage categories based on the primary tumor size (T), the regional lymph nodes status (N), and if there is any distant metastasis (M).[30] The most widely used TNM system is that of the American Joint Committee on Cancer.
Primary Tumor (T)
Tis: Carcinoma in-situ, Paget Disease With no Tumor
- T1: <2 cmT1a: 0.1 to 0.5 cmT1b: 0.5 to 1.0 cmT1c: 1.0 to 2.0 cm
- T2: 2 to 5 cm
- T3: >5 cm
- T4T4a: Chest wall involvementT4b: Skin involvementT4c: Both 4a and 4bT4d: Inflammatory ca
Regional Lymph Nodes (N)
- N1: Mobile ipsilateral axillary nodes
- N2: Fixed/matted ipsilateral axillary nodes
- N3N3a: Ipsilateral infraclavicular nodesN3b: Ipsilateral mammary nodesN3c: Ipsilateral supraclavicular nodes
Distant Metastases (M)
M1: Distant metastases
Breast Cancer Staging
Stage 0 comprises ductal carcinoma in situ (DCIS) and noninvasive breast cancer. Early invasive cancer includes stages I, IIa, and IIb. Stages IIIa, IIIb, and IIIc primarily involve locally advanced disease. Stage IV is all metastatic breast cancer.[68] (see Image. Breast Cancer Metastasis Sites)
Prognosis
The prognosis of breast cancer depends on the stage. Stage 0 and Stage I both have a 100% 5-year survival rate. The 5-year survival rate of Stage II and Stage III breast cancer is about 93% and 72%, respectively. When the disease spreads systemically, its prognosis worsens dramatically. Only 22% of Stage IV breast cancer patients will survive their next 5 years.[30]
Complications
Complications can arise from the treatment, whether chemotherapy, radiation, hormonal therapy, or surgery.
Surgical
- Infection
- Pain
- Bleeding
- Cosmetic issues
- Permanent scarring
- Alteration or loss of sensation in the chest area and reconstructed breasts
Chemotherapy
- Nausea/vomiting and diarrhea
- Hair loss
- Memory loss "chemo brain"
- Vaginal dryness
- Menopausal symptoms/fertility issues
- Neuropathy
Hormonal Therapy
- Hot flashes
- Vaginal discharge dryness
- Fatigue
- Nausea
- Impotence in males with breast cancer
Radiation
Deterrence and Patient Education
Breast cancer is the most commonly diagnosed cancer in women. Addressing the environmental and personal factors that increase the risk of breast cancer is vital in reducing breast cancer incidence. Screening helps detect premalignant lesions and breast cancer before it is clinically evident. Early detection leads to improved survival. Identifying patients at high risk for breast cancer is also crucial, as these individuals need to be monitored closely. Mammography, ultrasound, and MRI may be used for screening and diagnosis. A biopsy with histopathology and molecular markers should be performed on all patients. Early breast cancer is typically treated with breast conservation surgery, radiation, chemotherapy, or hormonal therapy. More advanced tumors require a mix of different modalities to obtain the best outcome. Long-term surveillance and compliance with therapy help improve survival.
Enhancing Healthcare Team Outcomes
Patient-centered care for individuals with breast requires collaboration among healthcare professionals, including physicians, advanced practice clinicians, nurses, pharmacists, and others. These neoplasms are often discovered during screening. The necessary skills involve interpreting radiological findings, identifying potential complications, effectively communicating these findings to the patient and their care team, and understanding the intricacies of breasts. Medical oncology, interventional radiology, pathology, general surgery, plastic surgery, and primary care practitioners typically play a role in coordinating and delivering care to patients with breast cancer. The entire healthcare team also plays a crucial role in ensuring that patients continue on surveillance pathways.
Media
(Click Image to Enlarge)
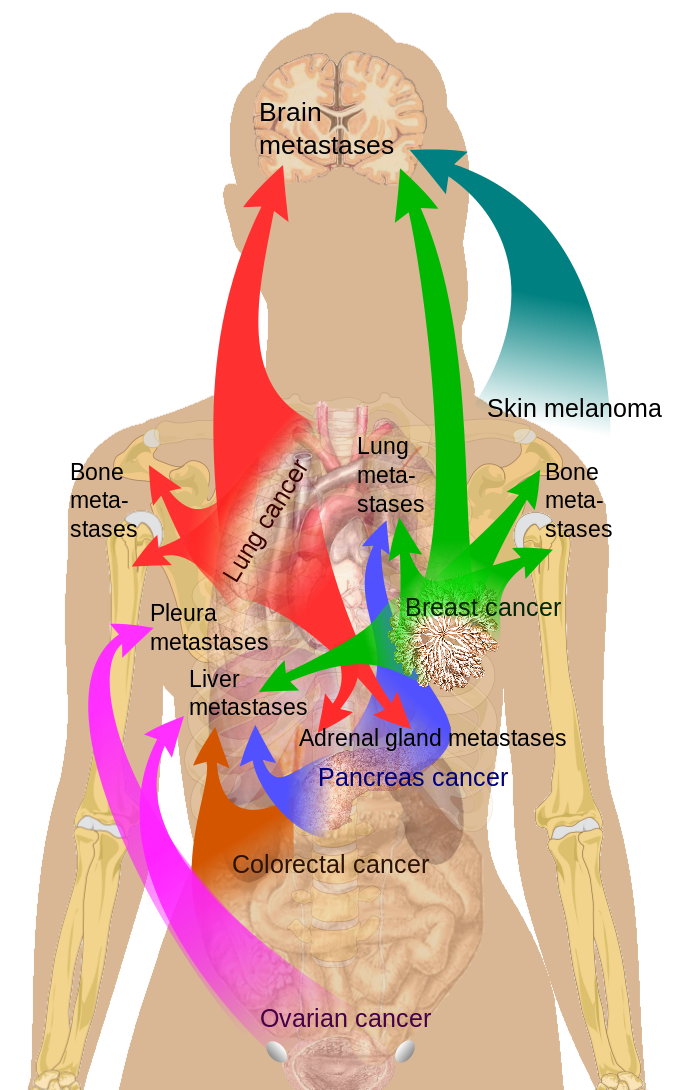
Common Sites of Breast Cancer Metastasis.
Medical Gallery of Mikael Häggström, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
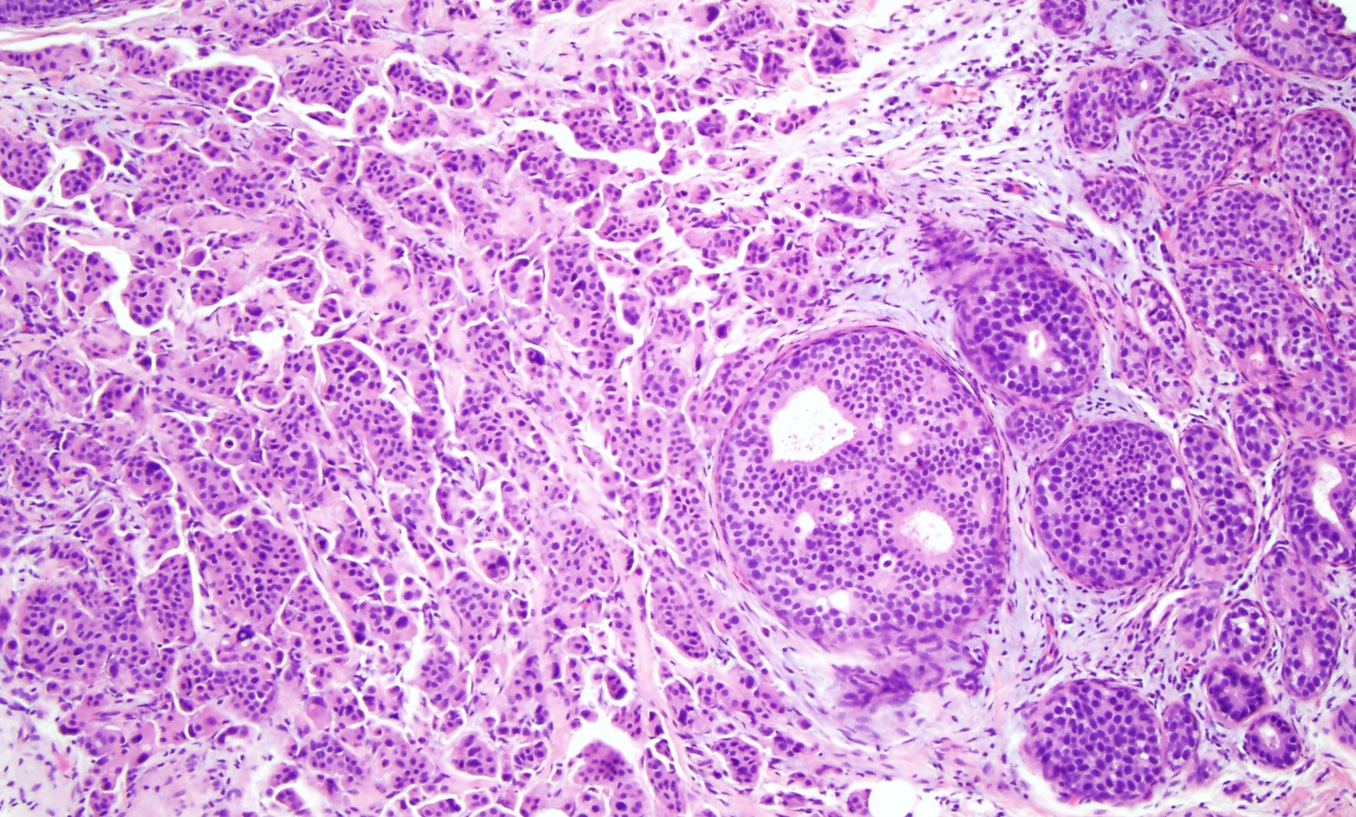
Invasive Ductal Carcinoma. Histological slide of high-grade ductal carcinoma in situ with invasive ductal carcinoma (×10). The left side of the image shows a sheet of cells with pleomorphic nuclei, arranged in tubules, infiltrating into the breast stroma, consistent with invasive ductal carcinoma originating from the adjacent high-grade ductal carcinoma in situ on the right side of the image.
Contributed by M Khan, DO
References
Watkins EJ. Overview of breast cancer. JAAPA : official journal of the American Academy of Physician Assistants. 2019 Oct:32(10):13-17. doi: 10.1097/01.JAA.0000580524.95733.3d. Epub [PubMed PMID: 31513033]
Level 3 (low-level) evidenceAlex A, Bhandary E, McGuire KP. Anatomy and Physiology of the Breast during Pregnancy and Lactation. Advances in experimental medicine and biology. 2020:1252():3-7. doi: 10.1007/978-3-030-41596-9_1. Epub [PubMed PMID: 32816256]
Level 3 (low-level) evidenceKashyap D, Pal D, Sharma R, Garg VK, Goel N, Koundal D, Zaguia A, Koundal S, Belay A. Global Increase in Breast Cancer Incidence: Risk Factors and Preventive Measures. BioMed research international. 2022:2022():9605439. doi: 10.1155/2022/9605439. Epub 2022 Apr 18 [PubMed PMID: 35480139]
PDQ Screening and Prevention Editorial Board. Breast Cancer Screening (PDQ®): Health Professional Version. PDQ Cancer Information Summaries. 2002:(): [PubMed PMID: 26389344]
Doren A, Vecchiola A, Aguirre B, Villaseca P. Gynecological-endocrinological aspects in women carriers of BRCA1/2 gene mutations. Climacteric : the journal of the International Menopause Society. 2018 Dec:21(6):529-535. doi: 10.1080/13697137.2018.1514006. Epub 2018 Oct 8 [PubMed PMID: 30295091]
Level 2 (mid-level) evidenceSung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a cancer journal for clinicians. 2021 May:71(3):209-249. doi: 10.3322/caac.21660. Epub 2021 Feb 4 [PubMed PMID: 33538338]
Parada H Jr, Sun X, Tse CK, Olshan AF, Troester MA. Lifestyle Patterns and Survival Following Breast Cancer in the Carolina Breast Cancer Study. Epidemiology (Cambridge, Mass.). 2019 Jan:30(1):83-92. doi: 10.1097/EDE.0000000000000933. Epub [PubMed PMID: 30299404]
White AJ, Bradshaw PT, Hamra GB. Air pollution and Breast Cancer: A Review. Current epidemiology reports. 2018 Jun:5(2):92-100. doi: 10.1007/s40471-018-0143-2. Epub 2018 Mar 27 [PubMed PMID: 30271702]
Gucalp A, Traina TA, Eisner JR, Parker JS, Selitsky SR, Park BH, Elias AD, Baskin-Bey ES, Cardoso F. Male breast cancer: a disease distinct from female breast cancer. Breast cancer research and treatment. 2019 Jan:173(1):37-48. doi: 10.1007/s10549-018-4921-9. Epub 2018 Sep 28 [PubMed PMID: 30267249]
Azamjah N, Soltan-Zadeh Y, Zayeri F. Global Trend of Breast Cancer Mortality Rate: A 25-Year Study. Asian Pacific journal of cancer prevention : APJCP. 2019 Jul 1:20(7):2015-2020. doi: 10.31557/APJCP.2019.20.7.2015. Epub 2019 Jul 1 [PubMed PMID: 31350959]
Collaborative Group on Hormonal Factors in Breast Cancer. Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease. Lancet (London, England). 2001 Oct 27:358(9291):1389-99 [PubMed PMID: 11705483]
Level 2 (mid-level) evidenceRoulot A, Héquet D, Guinebretière JM, Vincent-Salomon A, Lerebours F, Dubot C, Rouzier R. Tumoral heterogeneity of breast cancer. Annales de biologie clinique. 2016 Dec 1:74(6):653-660 [PubMed PMID: 27848916]
Prat A, Pineda E, Adamo B, Galván P, Fernández A, Gaba L, Díez M, Viladot M, Arance A, Muñoz M. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast (Edinburgh, Scotland). 2015 Nov:24 Suppl 2():S26-35. doi: 10.1016/j.breast.2015.07.008. Epub 2015 Aug 5 [PubMed PMID: 26253814]
Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lønning PE, Børresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000 Aug 17:406(6797):747-52 [PubMed PMID: 10963602]
McCart Reed AE, Kalinowski L, Simpson PT, Lakhani SR. Invasive lobular carcinoma of the breast: the increasing importance of this special subtype. Breast cancer research : BCR. 2021 Jan 7:23(1):6. doi: 10.1186/s13058-020-01384-6. Epub 2021 Jan 7 [PubMed PMID: 33413533]
Roux P, Knight S, Cohen M, Classe JM, Mazouni C, Chauvet MP, Reyal F, Colombo PE, Jouve E, Chopin N, Daraï E, Coutant C, Lambaudie E, Houvenaeghel G. Tubular and mucinous breast cancer: results of a cohort of 917 patients. Tumori. 2019 Feb:105(1):55-62. doi: 10.1177/0300891618811282. Epub 2018 Dec 20 [PubMed PMID: 30900967]
Cserni G. Histological type and typing of breast carcinomas and the WHO classification changes over time. Pathologica. 2020 Mar:112(1):25-41. doi: 10.32074/1591-951X-1-20. Epub [PubMed PMID: 32202537]
. Practice Bulletin Number 179: Breast Cancer Risk Assessment and Screening in Average-Risk Women. Obstetrics and gynecology. 2017 Jul:130(1):e1-e16. doi: 10.1097/AOG.0000000000002158. Epub [PubMed PMID: 28644335]
Baines CJ. Physical examination of the breasts in screening for breast cancer. Journal of gerontology. 1992 Nov:47 Spec No():63-7 [PubMed PMID: 1430885]
Menta A, Fouad TM, Lucci A, Le-Petross H, Stauder MC, Woodward WA, Ueno NT, Lim B. Inflammatory Breast Cancer: What to Know About This Unique, Aggressive Breast Cancer. The Surgical clinics of North America. 2018 Aug:98(4):787-800. doi: 10.1016/j.suc.2018.03.009. Epub 2018 May 24 [PubMed PMID: 30005774]
Henderson JA, Duffee D, Ferguson T. Breast Examination Techniques. StatPearls. 2025 Jan:(): [PubMed PMID: 29083747]
Jackson VP. Diagnostic mammography. Radiologic clinics of North America. 2004 Sep:42(5):853-70, vi [PubMed PMID: 15337421]
Mann RM, Kuhl CK, Moy L. Contrast-enhanced MRI for breast cancer screening. Journal of magnetic resonance imaging : JMRI. 2019 Aug:50(2):377-390. doi: 10.1002/jmri.26654. Epub 2019 Jan 18 [PubMed PMID: 30659696]
Nielsen S, Narayan AK. Breast Cancer Screening Modalities, Recommendations, and Novel Imaging Techniques. The Surgical clinics of North America. 2023 Feb:103(1):63-82. doi: 10.1016/j.suc.2022.08.004. Epub 2022 Oct 17 [PubMed PMID: 36410354]
Magny SJ, Shikhman R, Keppke AL. Breast Imaging Reporting and Data System. StatPearls. 2024 Jan:(): [PubMed PMID: 29083600]
Radovic N, Ivanac G, Divjak E, Biondic I, Bulum A, Brkljacic B. Evaluation of Breast Cancer Morphology Using Diffusion-Weighted and Dynamic Contrast-Enhanced MRI: Intermethod and Interobserver Agreement. Journal of magnetic resonance imaging : JMRI. 2019 May:49(5):1381-1390. doi: 10.1002/jmri.26332. Epub 2018 Oct 16 [PubMed PMID: 30325549]
Pediconi F, Marzocca F, Cavallo Marincola B, Napoli A. MRI-guided treatment in the breast. Journal of magnetic resonance imaging : JMRI. 2018 Dec:48(6):1479-1488. doi: 10.1002/jmri.26282. Epub 2018 Oct 14 [PubMed PMID: 30318672]
Watanabe Y, Anan K. The decision to perform or omit sentinel lymph node biopsy during mastectomy for ductal carcinoma in situ should be tailored in accordance with preoperative findings. Breast cancer (Tokyo, Japan). 2019 Mar:26(2):261-262. doi: 10.1007/s12282-018-0917-x. Epub 2018 Oct 16 [PubMed PMID: 30328007]
Verma P, Sharma R, Sharma N, Gulati A, Parashar A, Kaundal A. Fine-Needle Aspiration Cytology versus Core-Needle Biopsy for Breast Lesions: A Dilemma of Superiority between the Two. Acta cytologica. 2021:65(5):411-416. doi: 10.1159/000517005. Epub 2021 Jun 30 [PubMed PMID: 34192704]
Gradishar WJ, Moran MS, Abraham J, Aft R, Agnese D, Allison KH, Anderson B, Burstein HJ, Chew H, Dang C, Elias AD, Giordano SH, Goetz MP, Goldstein LJ, Hurvitz SA, Isakoff SJ, Jankowitz RC, Javid SH, Krishnamurthy J, Leitch M, Lyons J, Mortimer J, Patel SA, Pierce LJ, Rosenberger LH, Rugo HS, Sitapati A, Smith KL, Smith ML, Soliman H, Stringer-Reasor EM, Telli ML, Ward JH, Wisinski KB, Young JS, Burns J, Kumar R. Breast Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network : JNCCN. 2022 Jun:20(6):691-722. doi: 10.6004/jnccn.2022.0030. Epub [PubMed PMID: 35714673]
Level 1 (high-level) evidenceKerr AJ, Dodwell D, McGale P, Holt F, Duane F, Mannu G, Darby SC, Taylor CW. Adjuvant and neoadjuvant breast cancer treatments: A systematic review of their effects on mortality. Cancer treatment reviews. 2022 Apr:105():102375. doi: 10.1016/j.ctrv.2022.102375. Epub 2022 Mar 4 [PubMed PMID: 35367784]
Level 1 (high-level) evidenceShien T, Iwata H. Adjuvant and neoadjuvant therapy for breast cancer. Japanese journal of clinical oncology. 2020 Mar 9:50(3):225-229. doi: 10.1093/jjco/hyz213. Epub [PubMed PMID: 32147701]
Waks AG, Winer EP. Breast Cancer Treatment: A Review. JAMA. 2019 Jan 22:321(3):288-300. doi: 10.1001/jama.2018.19323. Epub [PubMed PMID: 30667505]
Czajka ML, Pfeifer C. Breast Cancer Surgery. StatPearls. 2024 Jan:(): [PubMed PMID: 31971717]
Goethals A, Rose J. Mastectomy. StatPearls. 2024 Jan:(): [PubMed PMID: 30855800]
Sevensma KE, Lewis CR. Axillary Sentinel Lymph Node Biopsy. StatPearls. 2023 Jan:(): [PubMed PMID: 31985977]
Toomey AE, Lewis CR. Axillary Lymphadenectomy. StatPearls. 2024 Jan:(): [PubMed PMID: 32491796]
Wapnir IL, Dignam JJ, Fisher B, Mamounas EP, Anderson SJ, Julian TB, Land SR, Margolese RG, Swain SM, Costantino JP, Wolmark N. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. Journal of the National Cancer Institute. 2011 Mar 16:103(6):478-88. doi: 10.1093/jnci/djr027. Epub 2011 Mar 11 [PubMed PMID: 21398619]
Level 2 (mid-level) evidenceLitière S, Werutsky G, Fentiman IS, Rutgers E, Christiaens MR, Van Limbergen E, Baaijens MH, Bogaerts J, Bartelink H. Breast conserving therapy versus mastectomy for stage I-II breast cancer: 20 year follow-up of the EORTC 10801 phase 3 randomised trial. The Lancet. Oncology. 2012 Apr:13(4):412-9. doi: 10.1016/S1470-2045(12)70042-6. Epub 2012 Feb 27 [PubMed PMID: 22373563]
Level 1 (high-level) evidenceWang X, Xu L, Yin Z, Wang D, Wang Q, Xu K, Zhao J, Zhao L, Yuan Z, Wang P. Locoregional recurrence-associated factors and risk-adapted postmastectomy radiotherapy for breast cancer staged in cT1-2N0-1 after neoadjuvant chemotherapy. Cancer management and research. 2018:10():4105-4112. doi: 10.2147/CMAR.S173628. Epub 2018 Oct 2 [PubMed PMID: 30323666]
Tang L, Matsushita H, Jingu K. Controversial issues in radiotherapy after breast-conserving surgery for early breast cancer in older patients: a systematic review. Journal of radiation research. 2018 Nov 1:59(6):789-793. doi: 10.1093/jrr/rry071. Epub [PubMed PMID: 30321392]
Level 1 (high-level) evidenceKirby AM. Updated ASTRO guidelines on accelerated partial breast irradiation (APBI): to whom can we offer APBI outside a clinical trial? The British journal of radiology. 2018 May:91(1085):20170565. doi: 10.1259/bjr.20170565. Epub 2018 Mar 23 [PubMed PMID: 29513031]
Vicini FA, Cecchini RS, White JR, Arthur DW, Julian TB, Rabinovitch RA, Kuske RR, Ganz PA, Parda DS, Scheier MF, Winter KA, Paik S, Kuerer HM, Vallow LA, Pierce LJ, Mamounas EP, McCormick B, Costantino JP, Bear HD, Germain I, Gustafson G, Grossheim L, Petersen IA, Hudes RS, Curran WJ Jr, Bryant JL, Wolmark N. Long-term primary results of accelerated partial breast irradiation after breast-conserving surgery for early-stage breast cancer: a randomised, phase 3, equivalence trial. Lancet (London, England). 2019 Dec 14:394(10215):2155-2164. doi: 10.1016/S0140-6736(19)32514-0. Epub 2019 Dec 5 [PubMed PMID: 31813636]
Level 1 (high-level) evidenceBartelink H, Horiot JC, Poortmans PM, Struikmans H, Van den Bogaert W, Fourquet A, Jager JJ, Hoogenraad WJ, Oei SB, Wárlám-Rodenhuis CC, Pierart M, Collette L. Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10-year results of the randomized boost versus no boost EORTC 22881-10882 trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007 Aug 1:25(22):3259-65 [PubMed PMID: 17577015]
Level 1 (high-level) evidenceOvergaard M, Nielsen HM, Tramm T, Højris I, Grantzau TL, Alsner J, Offersen BV, Overgaard J, DBCG Radiotherapy Group. Postmastectomy radiotherapy in high-risk breast cancer patients given adjuvant systemic therapy. A 30-year long-term report from the Danish breast cancer cooperative group DBCG 82bc trial. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2022 May:170():4-13. doi: 10.1016/j.radonc.2022.03.008. Epub 2022 Mar 11 [PubMed PMID: 35288227]
Donker M, van Tienhoven G, Straver ME, Meijnen P, van de Velde CJ, Mansel RE, Cataliotti L, Westenberg AH, Klinkenbijl JH, Orzalesi L, Bouma WH, van der Mijle HC, Nieuwenhuijzen GA, Veltkamp SC, Slaets L, Duez NJ, de Graaf PW, van Dalen T, Marinelli A, Rijna H, Snoj M, Bundred NJ, Merkus JW, Belkacemi Y, Petignat P, Schinagl DA, Coens C, Messina CG, Bogaerts J, Rutgers EJ. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. The Lancet. Oncology. 2014 Nov:15(12):1303-10. doi: 10.1016/S1470-2045(14)70460-7. Epub 2014 Oct 15 [PubMed PMID: 25439688]
Level 1 (high-level) evidenceWhelan TJ, Olivotto IA, Parulekar WR, Ackerman I, Chua BH, Nabid A, Vallis KA, White JR, Rousseau P, Fortin A, Pierce LJ, Manchul L, Chafe S, Nolan MC, Craighead P, Bowen J, McCready DR, Pritchard KI, Gelmon K, Murray Y, Chapman JA, Chen BE, Levine MN, MA.20 Study Investigators. Regional Nodal Irradiation in Early-Stage Breast Cancer. The New England journal of medicine. 2015 Jul 23:373(4):307-16. doi: 10.1056/NEJMoa1415340. Epub [PubMed PMID: 26200977]
Choi KH, Ahn SJ, Jeong JU, Yu M, Kim JH, Jeong BK, Lee JH, Kim SH, Lee JH. Postoperative radiotherapy with intensity-modulated radiation therapy versus 3-dimensional conformal radiotherapy in early breast cancer: A randomized clinical trial of KROG 15-03. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2021 Jan:154():179-186. doi: 10.1016/j.radonc.2020.09.043. Epub 2020 Sep 24 [PubMed PMID: 32980384]
Level 1 (high-level) evidencePignol JP, Truong P, Rakovitch E, Sattler MG, Whelan TJ, Olivotto IA. Ten years results of the Canadian breast intensity modulated radiation therapy (IMRT) randomized controlled trial. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2016 Dec:121(3):414-419. doi: 10.1016/j.radonc.2016.08.021. Epub 2016 Sep 13 [PubMed PMID: 27637858]
Level 1 (high-level) evidenceDarby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, Correa C, Cutter D, Gagliardi G, Gigante B, Jensen MB, Nisbet A, Peto R, Rahimi K, Taylor C, Hall P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. The New England journal of medicine. 2013 Mar 14:368(11):987-98. doi: 10.1056/NEJMoa1209825. Epub [PubMed PMID: 23484825]
Level 2 (mid-level) evidenceEpler GR, Kelly EM. Systematic review of postradiotherapy bronchiolitis obliterans organizing pneumonia in women with breast cancer. The oncologist. 2014 Dec:19(12):1216-26. doi: 10.1634/theoncologist.2014-0041. Epub 2014 Oct 31 [PubMed PMID: 25361622]
Level 1 (high-level) evidenceTaghian AG, Assaad SI, Niemierko A, Floyd SR, Powell SN. Is a reduction in radiation lung volume and dose necessary with paclitaxel chemotherapy for node-positive breast cancer? International journal of radiation oncology, biology, physics. 2005 Jun 1:62(2):386-91 [PubMed PMID: 15890579]
Level 2 (mid-level) evidenceWilliams NR, Williams S, Kanapathy M, Naderi N, Vavourakis V, Mosahebi A. Radiation-induced fibrosis in breast cancer: A protocol for an observational cross-sectional pilot study for personalised risk estimation and objective assessment. International journal of surgery protocols. 2019:14():9-13. doi: 10.1016/j.isjp.2019.02.002. Epub 2019 Feb 13 [PubMed PMID: 31851743]
Level 2 (mid-level) evidenceCollette S, Collette L, Budiharto T, Horiot JC, Poortmans PM, Struikmans H, Van den Bogaert W, Fourquet A, Jager JJ, Hoogenraad W, Mueller RP, Kurtz J, Morgan DA, Dubois JB, Salamon E, Mirimanoff R, Bolla M, Van der Hulst M, Wárlám-Rodenhuis CC, Bartelink H, EORTC Radiation Oncology Group. Predictors of the risk of fibrosis at 10 years after breast conserving therapy for early breast cancer: a study based on the EORTC Trial 22881-10882 'boost versus no boost'. European journal of cancer (Oxford, England : 1990). 2008 Nov:44(17):2587-99. doi: 10.1016/j.ejca.2008.07.032. Epub 2008 Aug 29 [PubMed PMID: 18757193]
Level 1 (high-level) evidenceJacobson G, Bhatia S, Smith BJ, Button AM, Bodeker K, Buatti J. Randomized trial of pentoxifylline and vitamin E vs standard follow-up after breast irradiation to prevent breast fibrosis, evaluated by tissue compliance meter. International journal of radiation oncology, biology, physics. 2013 Mar 1:85(3):604-8. doi: 10.1016/j.ijrobp.2012.06.042. Epub 2012 Jul 28 [PubMed PMID: 22846413]
Level 1 (high-level) evidenceGross JP, Whelan TJ, Parulekar WR, Chen BE, Rademaker AW, Helenowski IB, Donnelly ED, Strauss JB. Development and Validation of a Nomogram to Predict Lymphedema After Axillary Surgery and Radiation Therapy in Women With Breast Cancer From the NCIC CTG MA.20 Randomized Trial. International journal of radiation oncology, biology, physics. 2019 Sep 1:105(1):165-173. doi: 10.1016/j.ijrobp.2019.05.002. Epub 2019 May 11 [PubMed PMID: 31085285]
Level 1 (high-level) evidenceDiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. The Lancet. Oncology. 2013 May:14(6):500-15. doi: 10.1016/S1470-2045(13)70076-7. Epub 2013 Mar 27 [PubMed PMID: 23540561]
Level 1 (high-level) evidenceBartels SAL, Donker M, Poncet C, Sauvé N, Straver ME, van de Velde CJH, Mansel RE, Blanken C, Orzalesi L, Klinkenbijl JHG, van der Mijle HCJ, Nieuwenhuijzen GAP, Veltkamp SC, van Dalen T, Marinelli A, Rijna H, Snoj M, Bundred NJ, Merkus JWS, Belkacemi Y, Petignat P, Schinagl DAX, Coens C, van Tienhoven G, van Duijnhoven F, Rutgers EJT. Radiotherapy or Surgery of the Axilla After a Positive Sentinel Node in Breast Cancer: 10-Year Results of the Randomized Controlled EORTC 10981-22023 AMAROS Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2023 Apr 20:41(12):2159-2165. doi: 10.1200/JCO.22.01565. Epub 2022 Nov 16 [PubMed PMID: 36383926]
Level 1 (high-level) evidencePierce SM, Recht A, Lingos TI, Abner A, Vicini F, Silver B, Herzog A, Harris JR. Long-term radiation complications following conservative surgery (CS) and radiation therapy (RT) in patients with early stage breast cancer. International journal of radiation oncology, biology, physics. 1992:23(5):915-23 [PubMed PMID: 1639653]
Level 2 (mid-level) evidenceMeric F, Buchholz TA, Mirza NQ, Vlastos G, Ames FC, Ross MI, Pollock RE, Singletary SE, Feig BW, Kuerer HM, Newman LA, Perkins GH, Strom EA, McNeese MD, Hortobagyi GN, Hunt KK. Long-term complications associated with breast-conservation surgery and radiotherapy. Annals of surgical oncology. 2002 Jul:9(6):543-9 [PubMed PMID: 12095969]
Grantzau T, Overgaard J. Risk of second non-breast cancer after radiotherapy for breast cancer: a systematic review and meta-analysis of 762,468 patients. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2015 Jan:114(1):56-65. doi: 10.1016/j.radonc.2014.10.004. Epub 2014 Nov 7 [PubMed PMID: 25454172]
Level 2 (mid-level) evidenceYe JC, Yan W, Christos P, Nori D, Chao KS, Ravi A. Second cancer, breast cancer, and cardiac mortality in stage T1aN0 breast cancer patients with or without external beam radiation therapy: a national registry study. Clinical breast cancer. 2015 Feb:15(1):54-9. doi: 10.1016/j.clbc.2014.07.003. Epub 2014 Aug 15 [PubMed PMID: 25223278]
Dracham CB, Shankar A, Madan R. Radiation induced secondary malignancies: a review article. Radiation oncology journal. 2018 Jun:36(2):85-94. doi: 10.3857/roj.2018.00290. Epub 2018 Jun 29 [PubMed PMID: 29983028]
Burguin A, Diorio C, Durocher F. Breast Cancer Treatments: Updates and New Challenges. Journal of personalized medicine. 2021 Aug 19:11(8):. doi: 10.3390/jpm11080808. Epub 2021 Aug 19 [PubMed PMID: 34442452]
Amjad MT, Chidharla A, Kasi A. Cancer Chemotherapy. StatPearls. 2024 Jan:(): [PubMed PMID: 33232037]
Syed YY. Oncotype DX Breast Recurrence Score(®): A Review of its Use in Early-Stage Breast Cancer. Molecular diagnosis & therapy. 2020 Oct:24(5):621-632. doi: 10.1007/s40291-020-00482-7. Epub [PubMed PMID: 32613290]
Andre F, Ismaila N, Allison KH, Barlow WE, Collyar DE, Damodaran S, Henry NL, Jhaveri K, Kalinsky K, Kuderer NM, Litvak A, Mayer EL, Pusztai L, Raab R, Wolff AC, Stearns V. Biomarkers for Adjuvant Endocrine and Chemotherapy in Early-Stage Breast Cancer: ASCO Guideline Update. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2022 Jun 1:40(16):1816-1837. doi: 10.1200/JCO.22.00069. Epub 2022 Apr 19 [PubMed PMID: 35439025]
Trayes KP, Cokenakes SEH. Breast Cancer Treatment. American family physician. 2021 Aug 1:104(2):171-178 [PubMed PMID: 34383430]
Korde LA, Somerfield MR, Carey LA, Crews JR, Denduluri N, Hwang ES, Khan SA, Loibl S, Morris EA, Perez A, Regan MM, Spears PA, Sudheendra PK, Symmans WF, Yung RL, Harvey BE, Hershman DL. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2021 May 1:39(13):1485-1505. doi: 10.1200/JCO.20.03399. Epub 2021 Jan 28 [PubMed PMID: 33507815]
Wu YT, Xu Z, Zhang K, Wu JS, Li X, Arshad B, Li YC, Wang ZL, Li HY, Wu KN, Kong LQ. Efficacy and cardiac safety of the concurrent use of trastuzumab and anthracycline-based neoadjuvant chemotherapy for HER2-positive breast cancer: a systematic review and meta-analysis. Therapeutics and clinical risk management. 2018:14():1789-1797. doi: 10.2147/TCRM.S176214. Epub 2018 Sep 26 [PubMed PMID: 30310287]
Level 1 (high-level) evidenceDieci MV, Vernaci G, Guarneri V. Escalation and de-escalation in HER2 positive early breast cancer. Current opinion in oncology. 2019 Jan:31(1):35-42. doi: 10.1097/CCO.0000000000000492. Epub [PubMed PMID: 30325338]
Level 3 (low-level) evidenceYe F, Dewanjee S, Li Y, Jha NK, Chen ZS, Kumar A, Vishakha, Behl T, Jha SK, Tang H. Advancements in clinical aspects of targeted therapy and immunotherapy in breast cancer. Molecular cancer. 2023 Jul 6:22(1):105. doi: 10.1186/s12943-023-01805-y. Epub 2023 Jul 6 [PubMed PMID: 37415164]
Husinka L, Koerner PH, Miller RT, Trombatt W. Review of cyclin-dependent kinase 4/6 inhibitors in the treatment of advanced or metastatic breast cancer. Journal of drug assessment. 2020 Dec 18:10(1):27-34. doi: 10.1080/21556660.2020.1857103. Epub 2020 Dec 18 [PubMed PMID: 33414982]
Debien V, De Caluwé A, Wang X, Piccart-Gebhart M, Tuohy VK, Romano E, Buisseret L. Immunotherapy in breast cancer: an overview of current strategies and perspectives. NPJ breast cancer. 2023 Feb 13:9(1):7. doi: 10.1038/s41523-023-00508-3. Epub 2023 Feb 13 [PubMed PMID: 36781869]
Level 3 (low-level) evidenceLoibl S, Poortmans P, Morrow M, Denkert C, Curigliano G. Breast cancer. Lancet (London, England). 2021 May 8:397(10286):1750-1769. doi: 10.1016/S0140-6736(20)32381-3. Epub 2021 Apr 1 [PubMed PMID: 33812473]
Al-Hilli Z, Wilkerson A. Breast Surgery: Management of Postoperative Complications Following Operations for Breast Cancer. The Surgical clinics of North America. 2021 Oct:101(5):845-863. doi: 10.1016/j.suc.2021.06.014. Epub 2021 Aug 7 [PubMed PMID: 34537147]