Introduction
The adult human skeleton is composed of 206 bones. At birth, there are approximately 270 bones, with the final adult count decreasing as a portion of these bones fuse during phases of skeletal growth and maturation. Bone is a metabolically active connective tissue that provides structural support, facilitates movement, and protects vital organs; this tissue plays an important role in regulating mineral and acid-base balance homeostasis and also provides the environment for hematopoiesis (blood cell production) within the bone marrow. Bone is composed of an extracellular matrix and bone cells (osteocytes).[1][2]
Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Function
Bone Cells
Bone cells make up about 10% of total bone volume. There are 4 types of cells:
- Osteoprogenitor (stem) cells: Osteoprogenitor cells retain the ability to re-differentiate into osteoblasts. They reside in the bone canals, endosteum, periosteum, and marrow. They may regulate the influx and efflux of mineral ions into and out of the bone extracellular matrix. They also are responsible for the formation of bone remodeling compartments (BRC) with a specialized microenvironment.[3]
- Osteoblasts (bone-forming cells): They are tightly packed on the surface of the bone. They synthesize and secrete bone matrix (osteoid). They also regulate bone mineralization by secreting alkaline phosphatase (a marker for bone formation) and a set of proteins known as dentin matrix protein (DMP-1) and bone sialoprotein, which act as nucleators for mineralization. Osteocalcin and osteonectin are calcium and phosphate-binding proteins secreted by osteoblasts, which regulate the deposition of minerals by regulating the number of hydroxyapatite crystals. Osteoblasts ultimately have one of two fates: (1) remain quiescent osteoblasts lining cells or (2) become osteocytes. Osteoblasts regulate osteoclastogenesis (osteoclast formation) and osteocyte formation. Vitamin D and parathyroid hormone (PTH) stimulate osteoblasts to secrete macrophage cerebrospinal fluid (CSF) and express osteoprotegerin ligands, which are important for osteoclastogenesis (see Image. Osteoclastogenesis).[4]
- Osteocytes (mechanosensing cells): These account for 90% of all bone cells. They are derived from osteoblasts. They reside within the bone network known as the lacuna canalicular system. They do not normally express alkaline phosphatase but do express osteocalcin and other bone matrix proteins. They maintain a connection with each other and bone surfaces via their cytoplasmic processes. Osteocytes are linked metabolically and electrically through gap junctions. Their primary function is mechanosensation. Osteocytes detect mechanical loading through the physical deformation of bone matrix and fluid flow shear stress resulting from the flow of canalicular fluid through the lacuna canalicular network. Osteocytes act as orchestrators of bone remodeling and as a result, are also considered endocrine cells. They secrete fibroblast growth factor-23 (FGF23) to regulate serum phosphate levels. FGF23 decreases renal and intestinal sodium and phosphate co-transporter expression and subsequently increases renal phosphate excretion by both kidneys.[5]
- Osteoclasts (bone-resorbing cells): These are multinucleated cells that originated from mononuclear monocyte-macrophage cells. Receptor activator of nuclear factor kappa beta (RANKL) and macrophage CSF are 2 cytokines that are critical for osteoclast formation. They are important for osteoclast precursors to proliferate and differentiate into mature osteoclasts. Osteoprotegerin is a membrane-bound secreted protein that binds RANKL (see figure) to inhibit its action at the RANK receptor and subsequently inhibit osteoclastogenesis. Bone resorption depends on osteoclast secretion of hydrogen ions, tartrate-resistant acid phosphatase (TRAP), and cathepsin K enzymes. Hydrogen ions acidify the resorption compartment beneath osteoclasts to dissolve the mineral component of the bone matrix, whereas cathepsin K and TRAP digest the proteinaceous matrix, which is mostly composed of type I collagen. PTH stimulates osteoclast activity while calcitonin inhibits it.[6]
Bone Extracellular Matrix
The bone extracellular matrix makes up 90% of the overall bone volume and consists of inorganic (mineral) and organic matrices.
- Inorganic bone matrix accounts for 99% of the body's storage of calcium, 85% of the phosphorus, and 40% to 60% of the magnesium and sodium. It is mainly in the form of hydroxyapatite [Ca10(PO4)6(OH)2] to provide the bone its strength, stiffness, and resistance to compressive forces.
- Organic bone matrix is secreted by osteoblasts and is predominantly type I collagen; this also contains glycoproteins, growth factors, and proteoglycans. Growth factors (such as osteocalcin, osteonectin, and bone sialoprotein) play important roles in osteoid formation, mineralization, and bone remodeling. The organic matrix gives bone its form and provides resistance to tensile forces.[7]
Bone Remodeling
This is a physiological process in which old or damaged bone is removed by osteoclasts and then replaced by new bone formed by osteoblasts. Bone formation and resorption are tightly coupled to ensure no net change in bone mass or quality after each remodeling. Bone remodeling requires the coordinated action of the four types of bone cells.
The process involves 4 major distinct but overlapping phases:
- Phase 1: Initiation/activation of bone remodeling at a specific site. The osteoclast precursors are recruited to bone remodeling compartments (BRC).
- Phase 2: Bone resorption and concurrent recruitment of osteoprogenitors. Bone resorption represents the predominant event, but the recruitment of mesenchymal stem cells (MSCs) and/or osteoprogenitors into the BRC is also initiated.
- Phase 3: Osteoblast differentiation and function (osteoid synthesis). Excavated bone is replaced with osteoid produced by osteoblasts.
- Phase 4: Mineralization of osteoid and completion of bone remodeling. The osteoid is mineralized, and the bone remodeling cycle is concluded.[8]
Clinical Significance
Osteoporosis
Osteoporosis is a common disorder of bone remodeling, which is characterized by low bone mass and structural deterioration of bone, causing increased fragility and vulnerability to fractures. There are 2 types of osteoporosis:
Primary Osteoporosis
Type I (Postmenopausal Osteoporosis)
- Cause: a decline in estrogen levels associated with menopause
- Pathophysiology: estrogen deficiency causes an increase in osteoclast activity by increasing RANKL and M-CSF expression and inhibiting osteoclast apoptosis by reducing FasL expression by preosteoclasts
Type II (Age-Related Osteoporosis or Senile Osteoporosis)
- Cause: age-related and centered on osteoblasts (bone formation) in addition to bone resorption in postmenopausal women
- Pathophysiology: decreased bone formation in men and women is caused by changes in reactive oxygen species (ROS), insulin-like growth factor 1 (IGF-1), and PTH levels associated with aging
Glucocorticoid-induced Osteoporosis (Secondary Osteoporosis)
- Cause: Glucocorticoids are immunomodulatory drugs that are used to treat a variety of autoimmune disorders and inflammatory conditions such as rheumatoid arthritis and multiple sclerosis. Bone loss and increased risk of fractures are among the common side effects of glucocorticoid treatment.
- Pathophysiology: Glucocorticoids inhibit the differentiation of osteoprogenitors into osteoblasts and promote their differentiation into adipocytes (fat cells). They also increase osteoblast apoptosis and impair their functions. Additionally, glucocorticoids target mature osteoclasts to prolong their life span which worsens the imbalance between bone formation and bone resorption in favor of bone resorption.[9][10][11][12][13][14]
Media
(Click Image to Enlarge)
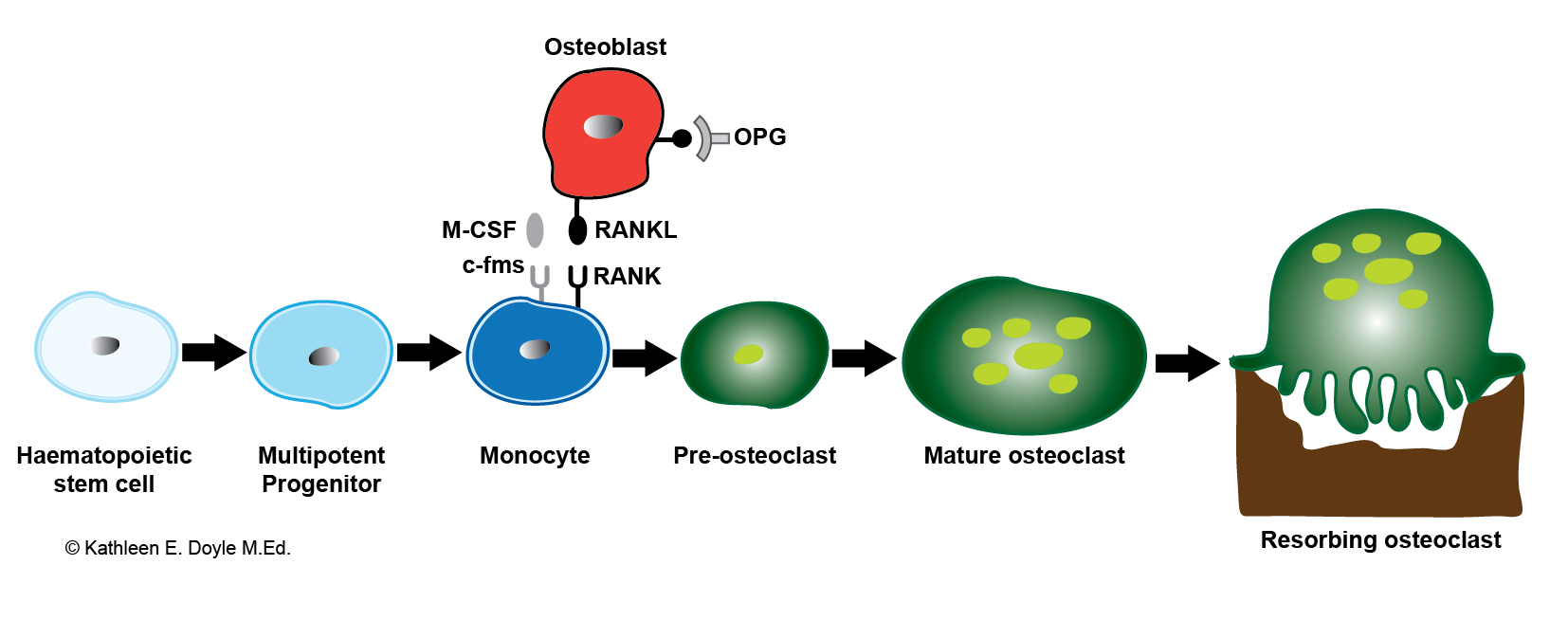
Osteoclastogenesis. Bone-resorbing osteoclasts originate from hemopoietic cells of the monocyte–macrophage lineage under the control of bone-forming osteoblasts. Illustration includes RANKL, the receptor activator of NF-κB ligand; M-CSF, macrophage colony-stimulating factor; and OPG, osteoprotegerin.
Contributed by KE Doyle, MEd
References
Clarke B. Normal bone anatomy and physiology. Clinical journal of the American Society of Nephrology : CJASN. 2008 Nov:3 Suppl 3(Suppl 3):S131-9. doi: 10.2215/CJN.04151206. Epub [PubMed PMID: 18988698]
Dallas SL, Prideaux M, Bonewald LF. The osteocyte: an endocrine cell ... and more. Endocrine reviews. 2013 Oct:34(5):658-90. doi: 10.1210/er.2012-1026. Epub 2013 Apr 23 [PubMed PMID: 23612223]
Corradetti B, Taraballi F, Powell S, Sung D, Minardi S, Ferrari M, Weiner BK, Tasciotti E. Osteoprogenitor cells from bone marrow and cortical bone: understanding how the environment affects their fate. Stem cells and development. 2015 May 1:24(9):1112-23. doi: 10.1089/scd.2014.0351. Epub 2015 Mar 6 [PubMed PMID: 25517215]
Level 3 (low-level) evidenceCaetano-Lopes J, Canhão H, Fonseca JE. Osteoblasts and bone formation. Acta reumatologica portuguesa. 2007 Apr-Jun:32(2):103-10 [PubMed PMID: 17572649]
Aarden EM, Burger EH, Nijweide PJ. Function of osteocytes in bone. Journal of cellular biochemistry. 1994 Jul:55(3):287-99 [PubMed PMID: 7962159]
Level 3 (low-level) evidenceBoyce BF, Yao Z, Xing L. Osteoclasts have multiple roles in bone in addition to bone resorption. Critical reviews in eukaryotic gene expression. 2009:19(3):171-80 [PubMed PMID: 19883363]
Level 3 (low-level) evidenceFeng X. Chemical and Biochemical Basis of Cell-Bone Matrix Interaction in Health and Disease. Current chemical biology. 2009 May 1:3(2):189-196 [PubMed PMID: 20161446]
Hadjidakis DJ, Androulakis II. Bone remodeling. Annals of the New York Academy of Sciences. 2006 Dec:1092():385-96 [PubMed PMID: 17308163]
Feng X, McDonald JM. Disorders of bone remodeling. Annual review of pathology. 2011:6():121-45. doi: 10.1146/annurev-pathol-011110-130203. Epub [PubMed PMID: 20936937]
Level 3 (low-level) evidenceHenriksen K, Bollerslev J, Everts V, Karsdal MA. Osteoclast activity and subtypes as a function of physiology and pathology--implications for future treatments of osteoporosis. Endocrine reviews. 2011 Feb:32(1):31-63. doi: 10.1210/er.2010-0006. Epub 2010 Sep 17 [PubMed PMID: 20851921]
Level 3 (low-level) evidenceVaracallo M, Seaman TJ, Jandu JS, Pizzutillo P. Osteopenia. StatPearls. 2024 Jan:(): [PubMed PMID: 29763053]
Varacallo M, Davis DD, Pizzutillo P. Osteoporosis in Spinal Cord Injuries. StatPearls. 2024 Jan:(): [PubMed PMID: 30252365]
Varacallo MA, Fox EJ. Osteoporosis and its complications. The Medical clinics of North America. 2014 Jul:98(4):817-31, xii-xiii. doi: 10.1016/j.mcna.2014.03.007. Epub 2014 May 9 [PubMed PMID: 24994054]
Varacallo MA, Fox EJ, Paul EM, Hassenbein SE, Warlow PM. Patients' response toward an automated orthopedic osteoporosis intervention program. Geriatric orthopaedic surgery & rehabilitation. 2013 Sep:4(3):89-98. doi: 10.1177/2151458513502039. Epub [PubMed PMID: 24319621]