Introduction
Benign prostatic hyperplasia (BPH) refers to the nonmalignant growth or hyperplasia of prostate tissue and is a common cause of lower urinary tract symptoms (LUTS) in older men. Disease prevalence has been shown to increase with advancing age. The histological prevalence of BPH at autopsy is as high as 50% to 60% for males in their 60s, increasing to 80% to 90% of those older than 70 years of age.[1]
Several definitions exist in the literature when describing BPH. These include bladder outlet obstruction, LUTS, and benign prostatic enlargement (BPE). BPH describes the histological changes, BPE refers to the increased size of the gland (usually secondary to BPH), and bladder outlet obstruction is defined as the blockage to urinary flow.[2][3] Those with BPE who present with bladder outlet obstruction are also termed benign prostatic obstruction.[4]
Lower urinary tract symptoms (LUTS) describe the urinary abnormalities shared by disorders affecting the bladder and prostate, typically caused by BPH. These terms have largely replaced those historically termed "prostatism."
The development of BPH is characterized by stromal and epithelial cell proliferation in the prostate transition zone, which surrounds the urethra. This leads to urethral compression and bladder outflow obstruction, which can result in clinical manifestations of LUTS, urinary retention, or infections due to incomplete bladder emptying.[5] Long-term, untreated disease can lead to the development of chronic high-pressure retention (a potentially life-threatening condition) and long-term or permanent changes to the bladder detrusor muscle.
BPH treatment options range from watchful waiting to various medical and surgical interventions. Risk factors may be divided into non-modifiable and modifiable. Other factors such as age, genetics, geographical location, and obesity have all been shown to influence the development of BPH.[6][7]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
BPH arises due to the loss of homeostasis between prostatic cellular proliferation and apoptosis or cell death. This imbalance favors cellular proliferation without intervention. The result is increased numbers of prostatic periurethral epithelial and stromal cells, which can be seen histopathologically.[5] The etiology of BPH is influenced by a wide variety of risk factors, in addition to the direct hormonal effects of testosterone on prostate tissue. Men who are castrated before puberty or who have an androgen-related disorder do not develop BPH.
There is conflicting data on the role of non-steroidal anti-inflammatory medications (NSAIDs) in promoting BPH, with some studies indicating a positive association and others discounting any association.[8][9][10] Allopurinol is somewhat protective for BPH, possibly secondary to reduced oxidative stress from hyperuricemia effects.[11]
Testicular androgens are required to develop BPH as dihydrotestosterone (DHT) promotes tissue growth and cellular proliferation by interacting directly with prostatic epithelium and stroma.[5][12] Testosterone is converted to DHT by 5-alpha-reductase 2 in prostatic stromal cells and accounts for 90% of total intraprostatic androgens.[7] DHT directly influences prostatic stromal and adjacent cells, which affect cellular proliferation and apoptosis.[13] Interestingly, there does not appear to be any relationship between testosterone or DHT levels and the development of symptomatic BPH.[14]
Risk Factors
Non-modifiable and modifiable risk factors also contribute to the development of BPH. These have been shown to include diabetes, diet, genetic factors, localized inflammation, obesity, and metabolic syndrome.[7]
- Diabetes and the use of antidiabetic medications, particularly insulin, appear to increase the risk of BPH, LUTS, and prostatic surgery.[15][16]
- Dietary factors also appear to influence the development of BPH. Beta-carotene, carotenoids, and vitamin A seem somewhat protective, while excessive alcohol ingestion, heavy caffeine intake, and high-dose supplemental vitamin C tend to increase BPH risk and symptoms.[17][18][19] No prepared dietary supplement has been proven to help BPH in properly performed, randomized, controlled studies.
- Genetic predisposition to BPH has been demonstrated in cohort studies. First-degree relatives in 1 study demonstrated a 4-fold increase in the risk of BPH compared to the control.[20] These findings have demonstrated consistency in twin studies looking at the disease severity of BPH, with higher rates of LUTS seen in monozygotic twins.[21][22]
- Localized inflammation is often associated with BPH, at least histologically.[23][24][25] While the exact etiology is unclear, possible causes include increased detrusor voiding pressure, obesity, low-grade or chronic prostatitis, compression of the prostatic ducts, and autoimmune disorders. This would suggest that the use of NSAIDs could be used to treat symptomatic BPH. Three randomized studies have confirmed that NSAIDs can improve BPH symptoms, but the difference was relatively modest at <3 points (International Prostate Symptom Scores [IPSS]) and <1 mL/s improvement in urine peak flow rate.[26]
- Obesity is associated with an increased risk of BPH in observational studies.[27][28] The exact cause is unclear but is likely multifactorial, as obesity makes up 1 aspect of metabolic syndrome. Proposed mechanisms include increased levels of systemic inflammation and higher levels of estrogens.[29][30]
- Metabolic syndrome refers to conditions that include hypertension, glucose intolerance/insulin resistance, and dyslipidemia. Meta-analysis has demonstrated those with metabolic syndrome and obesity have significantly higher prostate volumes.[31] Further studies looking at men with elevated glycosylated hemoglobin levels (Hba1c) have demonstrated an increased risk of LUTS.[15] Limitations of these studies are that there were no subsequent significant differences in prostate symptom scores, and the effect of diabetes on LUTS has been shown to be multifactorial.[31][32] Further studies are therefore required to establish causation in these individuals.
Epidemiology
Differences in definitions make the interpretation of population-based studies regarding BPH difficult. For example, BPH can refer to histology, prostate enlargement, prostatic glandular hypertrophy, bladder outlet obstruction, or just a physician's diagnosis of BPH. Lower urinary tract symptoms (LUTS) refer to the large variety of urinary symptoms shared by disorders affecting the prostate or bladder, which may or may not be due to BPH.
Age is a significant predictor of the development of BPH and subsequent LUTS. Fifty percent of men older than 50 show evidence of BPH, and the association with the development of LUTS increases linearly with age.[33][34] This is supported by studies that have demonstrated increases in prostate volume with age (2% to 2.5% increase in size per year).[35]
In the US, studies have shown BPH prevalence to be as high as 70% in those between 60 and 69 years of age and more than 80% in those over 70 years.[36] In a Boston area community health survey, the prevalence of male LUTS alone significantly increased with age from 8% (30 to 39 yrs) to 35% (60 to 69 yrs). Other US population-based studies have shown 56% of men between 50 and 79 years reported BPH symptoms.[37][38]
At a population level, the reported prevalence of BPH increased dramatically between 1998 and 2007 in the US, with the number of cases nearly doubling.[39] These increases are attributed to an aging population, with those older than 80 years projected to be 19.5 million in 2030 (up from 9.3 million in 2003).[40] As the worldwide population grows older, the number of symptomatic BPH cases is expected to rise.
International studies have suggested that Western populations have significantly higher prostate volumes than those from other parts of the world, particularly Southeast Asia.[41] Further studies looking at the correlation of prostate volume with LUTS found that lower prostate volumes did not necessarily correlate with symptoms, as higher mean IPSS was observed in a cohort of Indian men compared to similar Western populations.[42]
Pathophysiology
The development of LUTS and bladder outlet obstruction in men with BPH can be attributable to static and dynamic components.[43] Static obstruction is a direct consequence of prostate enlargement, resulting in periurethral compression and bladder outlet obstruction. Prostate enlargement distorts the bladder outlet, causing urinary obstruction, while periurethral compression requires increasing voiding pressures to overcome flow resistance.[44]
The prostate's median lobe can enlarge intravesically, where increased detrusor pressure will tend to close the bladder outlet and further restrict urination. If the median lobe enlarges unevenly, it can create a flap or "ball-valve" effect, closing the bladder outlet during voiding, resulting in significantly restricted flow and incomplete bladder emptying.
Dynamic components include the prostatic smooth muscle tension (hence using five alpha-reductase inhibitors to reduce prostate volume and alpha-blockers to relax prostatic smooth muscle).[45] This is explained by decreased elasticity and collagen in the prostatic urethra in men with BPH, which exacerbates symptoms of bladder outlet obstruction due to loss of compliance and increased flow resistance.[46] It also explains why prostate size alone is not always a reliable predictor of disease.
Although BPH can increase prostate-specific antigen levels (PSA), it is not a risk factor for prostate cancer.[47] BPH occurs primarily in the central/transitional portion of the prostate, while malignancies typically form in the prostatic periphery.
Histopathology
Microscopic histological examination demonstrates that BPH is a hyperplastic process with increased cell numbers, including both glandular and stromal cellular proliferation. Hyperplasia occurs both in the periurethral and transition zones. Specifically, periurethral zones demonstrate hyperplastic stromal nodules, whereas glandular nodular proliferation is seen within the transition zone.[48]
History and Physical
History
In the elective setting, a focused medical history should include all aspects of urinary symptomatology, including onset, timing, severity, exacerbating and relieving factors, and degree of bother.
Lower urinary tract symptoms (LUTS) can be divided into storage (frequency, nocturia, urgency) and voiding disorders (weak or intermittent stream, straining to void [stranguria], hesitancy, prolonged micturition, incomplete emptying) and can help establish other causes of urinary problems such as urinary tract infections (UTIs), overactive bladder, or neurogenicity, in addition to determining the affected organ (bladder vs prostate). Men with BPH are likely to report symptoms of nocturia, poor stream, hesitancy, or prolonged micturition.
Red flags help point to more sinister causes of urinary symptoms such as prostate cancer, spinal disorders such as cauda equina, or chronic high-pressure retention (which can lead to silent renal failure due to reflux).
A complete medication history should be taken, including any BPH drugs or supplements patients may have tried and their use of anticoagulants, which may affect surgical procedures.
The patient's overall fitness should also be established to determine suitability for future interventions (fitness for anesthesia, independence, exercise tolerance, and ability to complete activities of daily living). The symptom burden on quality of life should also be established.
Various non-urological conditions can cause or exacerbate urinary symptoms. For example:
- Diuretic use for congestive heart failure and hypertension can worsen urinary frequency and nocturia.[49][50]
- Neurological problems such as Parkinson's, stroke, cauda equina syndrome, multiple sclerosis, and various spinal cord disorders can affect the complex reflexes involved in bladder storage and voiding.[51][52][53]
- Poorly controlled diabetes causes polyuria due to osmotic diuresis and detrusor neuropathy, resulting in decreased bladder sensation, reduced contractility, and incomplete emptying.[54][55]
- Diabetes insipidus will cause increased urinary frequency and polyuria.[55][56]
Physical Examination
In the elective setting, the examination should include an abdominal examination (looking for palpable bladder/loin pain, lumps, hernias, or masses) and an examination of the external genitalia (meatal stenosis, testicular abnormalities, or phimosis). A neurological examination will help identify any neuropathy. The examination should include a digital rectal examination (DRE), making note of the particular size, shape, symmetry, nodularity, and consistency (smooth/hard) of the prostate. A smooth, enlarged prostate typically characterizes BPH.
Further evaluation includes the following: [57]
- Urinalysis
- Digital rectal examination
- Internation Prostate Symptom Score (IPSS) or the American Urological Association symptom (AUA) symptom score
- Postvoid residual volume (PVR) to determine whether the bladder is emptying adequately
- A frequency-volume chart or 24-hour voiding diary (optional)
- Peak flow test (optional)
- Laboratory evaluation for kidney function (BUN and creatinine) and diabetes (fasting glucose, Hgb A1c) if not previously performed.
- PSA test if appropriate (See our companion StatPearls reference article on "Prostate Cancer Screening.")[58]
Symptom Score Questionnaires
Both the AUA symptom score and the International Prostate Symptom Score (IPSS) have been verified and validated. They are used to identify significant LUTS, determine their type (obstructive or irritative), and assess their severity.[59] They are useful when quantifying symptom severity, tracking symptom relief with therapy, and categorizing patients for treatment.[7]
New male patients aged 50 or older can be given a simplified questionnaire to fill out while in the waiting room or after arrival in the examination room before the physician's entrance. A quick look at the symptom score will instantly identify urinary issues that should be addressed. Treatment is generally indicated when the symptom scores reach ≥10. The scoring can also be used after treatment to track symptom improvement.
The AUA symptom score (and the similar IPPS) uses the following questions.[59][60][61]
American Urological Association Symptom Score
The history of the patient presenting with symptoms of acute urinary retention should focus on LUTS using questions such as, "Over the past month, ..."
- How frequently have you had the sensation of not being able to empty your bladder completely after voiding?
- How frequently have you had to urinate again less than 2 hours after finishing urination?
- How frequently have you found you stopped and started several times when you were voiding?
- How often have you found it difficult to postpone urination?
- Over the past month, how often have you had a weak stream?
- How often have you had to push or strain to begin urination?
- How many times did you get up to urinate from the time you go to bed until you get up in the morning?
Responses are then scored according to the following:
- 0 = Not at all
- 1 = < 1 time in 5 (once in a while)
- 2 = < half the time
- 3 = About half the time
- 4 = > than half the time
- 5 = Almost always or all the time
The IPSS and AUA symptom scores categorize patients into 3 groups based on symptoms. The groups are mild (scores 0 to 9), moderate (scores 10 to 19), and severe (scores 20 to 35).
A symptom score ≥ 10 suggests that BPH treatment should be initiated, increased, or otherwise modified to provide additional relief. Those with more severe symptoms are less likely to benefit substantially from conservative or medical measures alone.
Evaluation
Standard investigation of BPH includes a urinalysis, IPSS or AUA symptom score, a DRE, PVR determination, and urine flow studies to establish if there is evidence of obstructive or irritative voiding. Further tests may be indicated depending on these results and the patient's history.
Urinalysis
Urine specimen testing can help detect infection, microscopic hematuria, or metabolic disorders (glycosuria). Leukocytes and nitrites are common findings associated with infection, while the presence of proteinuria may suggest an underlying renal disorder. The AUA BPH Guidelines also recommend a urinalysis.[57]
Blood Tests
Blood tests, including BUN and creatinine, are useful to establish baseline renal function. They can help support the diagnosis of renal failure or acute kidney injury in someone with chronic high-pressure or acute retention. A fasting glucose level or a Hgb A1c can identify diabetes.
24-Hour Voiding Diary
A 24-hour urinary voiding diary, where the patient measures and records the time and volume voided for a complete day, can be extremely helpful in evaluating urinary disorders, particularly nocturia. High urinary volumes at night are more consistent with nocturnal polyuria, while small volumes suggest bladder overactivity. (See our companion StatPearls reference article on "Nocturia.")[50]
Prostate-Specific Antigen (PSA)
Prostate-specific antigen testing is somewhat predictive of prostate volume.[62][63][64][65] Benign prostates of 35 cc size will typically generate a PSA of 1.5 ng/mL.[66] PSA testing is recommended where cancer is suspected (hard prostatic nodule, asymmetry, metastatic disease suspected) or a previous baseline PSA has been previously established. PSA testing is not a routine test done for BPH but is recommended before starting five alpha-reductase therapy or performing surgery to avoid missing a possible prostatic malignancy. (See our companion StatPearls reference articles on "Prostate Specific Antigen" and "Prostate Cancer Screening.")[58][67]
Postvoid Residual Volume
A postvoid residual urine volume is measured to determine how well the bladder empties after urination. This can be done with a bladder scan, formal bladder ultrasound, or a quick straight catheterization. The easiest way is to do a bladder scan if that is available. The normal PVR would be <100 to 150 mL, while >200 mL would be considered pathological. The measurement should be performed immediately after the patient voids but is still useful if done within 15 to 20 minutes. The postvoid residual measurement is an important and valuable determinant in evaluating and assessing BPH. It is also recommended by the AUA 2021 Guidelines on the Management of BPH before surgical intervention.[57]
While urology offices and hospitals typically have dedicated bladder scanners, most primary care facilities do not. Many experts recommend bladder scanners for primary care offices to measure PVR and diagnose urinary retention in patients with suprapubic pain, incontinence, urinary difficulty, or symptoms of BPH. A bladder scan model specifically designed for primary care offices can also screen patients for abdominal aortic aneurysms. This makes it very cost-effective even for small primary care offices.
Urinary Flow Studies (Flowmetry)
Urine flow studies (flowmetry) determine peak urinary flow rates. This can help establish whether there is objective evidence for urinary obstruction. The peak flow rate is the most significant measurement. A peak flow ≥ 13 cc/sec is considered acceptable. A flow test requires a volume of at least 150 cc to be considered valid. (If the peak flow ≥13 cc/sec, then the volume does not matter.)
Optimally, the postvoid residual measurement is made immediately after the flowmetry study. Decreased peak flow unrelated to inadequate volume is typically due to either obstruction (BPH, detrusor sphincter dyssynergia, urethral stricture, or bladder neck contracture) or detrusor hypotonicity. Flowmetry is recommended by the AUA 2021 Guidelines on Management of BPH before surgical intervention.[57]
Pressure/Flow Studies
A pressure/flow study is recommended in cases of abnormal urination where the diagnosis is uncertain or the benefit of surgical intervention is unclear. Noninvasive studies such as PVR measurements and flowmetry are certainly sufficient in most cases, but only a pressure/flow study can reliably determine the adequacy of detrusor muscular contractility and the presence of bladder outlet obstruction.[68][69] Most men with obstructive BPH will have a low urinary peak flow of <10 cc/sec with a normal or high detrusor voiding pressure.[68] Pressure/flow studies are recommended by the AUA 2021 Guidelines on Management of BPH before surgical intervention.[57]
Urodynamics
Urodynamic studies are used to see how the bladder empties and fills. They include pressure/flow studies but also evaluate sphincteric function and possible neurogenicity. Urodynamics can help further assess patients where the diagnosis is not certain or where a neurogenic/overactive bladder is suspected (ie, neurological conditions that may affect the bladder, detrusor sphincter dyssynergia, equivocal flow studies, unclear diagnoses, spinal cord injuries, sacral disorders, etc). Urodynamics are not required in most patients with BPH and are only indicated where there is doubt about urinary function and obstruction, even after simpler studies.
Renal Ultrasound
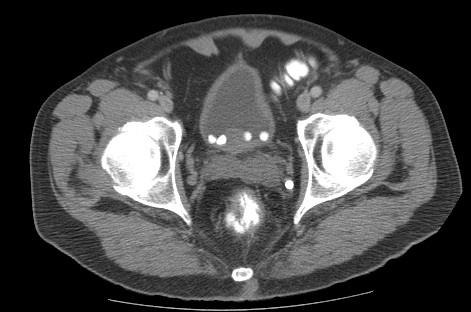
Renal ultrasound scans are used to look for evidence of hydronephrosis. They are only indicated in patients with high residual volumes, acute or chronic urinary retention, or unexplained renal impairment. Other indications include suspicion of urinary tract stones or to investigate unexplained hematuria. If nephrolithiasis or bladder calculi are suspected, a KUB x-ray or a noncontrast CT of the abdomen and pelvis may be used (see Image. CT of Pelvis Showing Multiple Bladder Stones).
Cystoscopy
Flexible cystoscopy should be used to investigate red-flag symptoms such as unexplained hematuria, possible bladder calculi, or suspected bladder cancer. It can also identify urethral strictures, evaluate median lobe enlargement, detect intravesical median lobe extensions and lobulations, determine the degree of obstruction in the prostatic urethra, and evaluate the bladder for stones and signs of damage. Cystoscopy provides a good estimate of prostate size and shape, can evaluate the degree of obstruction, and permits visualizing signs of bladder damage. It is strongly recommended before BPH surgical intervention. The bladder can be filled during the cystoscopy, allowing a urinary flow study and determining PVR.
Before surgical intervention for BPH, measurement of prostatic size is recommended, as many procedures have prostatic volume or shape limitations. While this is typically done by cystoscopy, it may also be accomplished by abdominal or transrectal ultrasonography, CT scan, or MRI.
Treatment / Management
Acute Urinary Retention
Men with BPH may present emergently with acute urinary retention or may be seen routinely in a primary care setting with chronic retention issues. Acute retention typically presents with significant lower abdominal or suprapubic pain, weak urinary stream or dribbling, and possibly incontinence from overflow.
Standard therapy for acute urinary retention from BPH is Foley catheter drainage, which immediately relieves the obstruction, reduces renal damage, eliminates patient discomfort, and allows for detrusor muscle recovery. The amount of urine drained immediately should be documented, as this reasonably predicts the bladder muscle's recovery potential.
The maximum bladder capacity is about 500 mL, so any amount over this is considered abnormal or pathological. Patients with acute retention with residual amounts up to 1000 mL will generally see a rapid recovery of bladder muscle tone. Recovery of detrusor muscle activity and tone is less likely as the residual volume increases, especially beyond 1500 mL to 2000 mL. In these cases, bladder muscle recovery is more problematic, and prolonged catheter drainage or self-intermittent clean catheterization may be required. There is also the immediate risk of post-obstructive diuresis. (See our companion StatPearls reference articles on "Postobstructive Diuresis" and "Male Urinary Retention: Acute and Chronic.")[61][70]
In addition to the Foley catheter, initial therapy usually includes treatment with an alpha-blocker. Such medical therapy should be initiated with alfuzosin (10 mg daily) or high-dose tamsulosin (0.8 mg) to maximize the success rate of a subsequent voiding trial. At least 72 hours should pass before a voiding trial is attempted to allow for the medication to reach full effect and the over-stretched detrusor muscle to recover. (5 alpha-reductase inhibitors are not used in the immediate treatment of acute urinary retention from BPH as they take months to be effective.)
Intractable urinary retention that is refractory to medical therapy generally requires surgery. International studies have demonstrated that BPH accounts for over two-thirds of all cases of acute urinary retention.[71] Men who experience acute urinary retention have a 15% chance of another episode in the future, with 75% of these individuals requiring surgery.[72](A1)
Chronic Urinary Retention
Chronic urinary retention may be either low or high pressure. High-pressure chronic retention commonly occurs over time in bladder outflow obstruction due to the elevated detrusor pressures required to overcome the outflow obstruction from BPH.[71] As bladder pressure increases, ureteral drainage into the bladder is impeded, resulting in bilateral hydronephrosis and renal functional deterioration. This can be identified by unexplained renal failure on blood tests and bilateral hydronephrosis on ultrasound or CT imaging studies. High-pressure retention is more likely associated with vesicoureteral reflux from the breakdown of the antireflux mechanism at the ureterovesical junction and nocturnal enuresis due to decreased bladder-neck muscle tone overnight.[71]
Immediate management consists of Foley catheterization. Some patients may develop post-obstructive diuresis immediately after catheter placement, especially if they have renal failure (elevated serum creatinine) or are fluid-overloaded. There is no benefit to clamping or restricting urinary drainage in these patients, and catheters should be allowed to drain freely.[73](A1)
Long-term management for recurrent or intractable acute retention and chronic urinary retention is usually a transurethral resection of the prostate (TURP) or similar surgery. Intermittent self-catheterization is an option in selected patients. Permanent Foley catheterization or suprapubic tube placement may also be appropriate in some situations.
Treatment of Benign Prostatic Hyperplasia
In men with symptomatic BPH, treatment options range from watchful waiting to medical and minimally invasive surgical interventions.[74] After a complete discussion of the available options and their respective risks and benefits, the type and nature of therapy will depend on the degree of "bother" or disease burden to the patient (as assessed by an IPSS or AUA symptom score).(B3)
Observation
Watchful waiting is a process of managing patients by giving lifestyle advice. Examples include weight loss, reducing caffeine intake, restricting fluid intake in the evening, implementing pelvic floor (Kegel) muscle exercises, and avoiding constipation to try and reduce risk factors and improve urinary symptoms. Patients should be involved in the discussion and informed of the risks of disease progression. Clinical progression has shown to be around 31% in 1 observational study, with 5% developing acute urinary retention.[75] These measures may be considered in those with mild symptoms.[7]
Medical Therapy
Both static and dynamic components contribute to the pathophysiology of BPH. Medical therapy aims to address both of these components.
Alpha-blockers are designed to minimize muscle tone in prostate stromal smooth muscle and bladder neck tissue. Alpha 1-adrenoreceptor blockage results in stromal smooth muscle relaxation addressing the dynamic component of BPH, thus improving flow and urinary symptoms. Typical improvement in IPSS and AUA symptoms scores is 4 to 6 points. Examples include selective alpha-blockers specifically designed for the prostate, such as tamsulosin (0.4 to 0.8 mg once daily), alfuzosin (10 mg once daily), and silodosin (4 to 8 mg once daily). Their effect maximizes in about 72 hours.
When used for patients with acute urinary retention, this suggests that the patient needs at least three days for the medication to reach peak efficacy before attempting a voiding trial. For this purpose, it is suggested that the 0.8 mg dose of tamsulosin or 10 mg of alfuzosin be used. If successful, the dosage can be reduced. When used for routine BPH symptoms, the 0.4 mg dose of tamsulosin can be tried first.
These medications are associated with floppy iris syndrome and should be used cautiously in patients requiring cataract or glaucoma surgery. Such situations should be discussed with the patient's ophthalmologist. Ejaculatory issues are a common side effect of alpha-blocker therapy, along with dizziness and low blood pressure. Patients with severe sulfa allergies may rarely react to tamsulosin, although most such patients can take the medication safely. Unlike five alpha-reductase inhibitors, alpha-blocker therapy does not appear to affect the long-term risk of acute urinary retention or the eventual need for surgical BPH management.[76]
Other alpha-blockers, such as terazosin and doxazosin, are equally effective in relieving prostatic issues but are much more likely to cause generalized side effects such as orthostatic hypotension. See the companion StatPearls reference review on "Alpha-Blockers" for more information.[77]
Tadalafil is a phosphodiesterase type 5 inhibitor similar to sildenafil that is indicated for erectile dysfunction (ED). It has also been found to be useful in treating symptoms of BPH and is roughly equivalent in efficacy to tamsulosin 0.4 mg.[78][79][80][81] Since it can treat both ED and BPH when given at the recommended 5 mg/day dosage, it can be particularly useful for patients requiring treatment for both conditions.[82][83] Combination therapy with daily tamsulosin 0.4 mg and tadalafil 5 mg has improved efficacy in treating BPH symptoms over monotherapy from either medication alone, although these results have been questioned, and side effects did increase.[84][85] Therefore, the 2021 AUA Management Guidelines for BPH do not recommend combination therapy.[57](A1)
Unlike tamsulosin, tadalafil does not risk an allergic reaction to sulfa or "floppy iris" syndrome, and ED has not been associated with its use.[86] Tadalafil can also be safely used with overactive bladder medications such as mirabegron.[87] Tadalafil is generic, relatively inexpensive, with few side effects, and is a very reasonable, if underutilized, therapy choice for patients with both BPH and ED. See the companion StatPearls reference review on "Tadalafil" for more information.[88]
Five alpha-reductase inhibitors, such as finasteride (5 mg once daily) and dutasteride (10 mg once daily), block the intraprostatic conversion of testosterone to DHT.[89]. This causes a reduction in individual cell volume and an increase in cellular apoptosis.[90] The overall effect is a reduction in prostatic tissue volume, although it takes several months to show noticeable improvement, with six months needed for maximal effectiveness.[7] As a result of treatment, serum PSA can be reduced by 50%, with prostate volume decreasing by up to 25%.(A1)
In general, only patients with prostates larger than 30 grams in size are likely to see any clinical benefit from this medication. Five alpha-reductase inhibitors have been shown to alter the disease process, prolong the alpha-blocker BPH medications' effectiveness, and delay surgical intervention.[76] While similar, dutasteride has a longer half-life and appears slightly more effective than finasteride.[91](A1)
Five alpha-reductase inhibitors can help reduce bleeding during and after prostate surgery if started 2 to 4 weeks earlier.[92] They are also useful for controlling prostatic bleeding unrelated to surgery and have been shown to reduce the risk of urinary retention and delay the need for BPH surgical intervention.(A1)
An alpha-blocker and a five alpha-reductase inhibitor are often combined to improve voiding symptoms. This is backed by studies confirming the effectiveness of combination therapy over monotherapy for symptomatic BPH.[93][94](A1)
The use of five alpha-reductase inhibitors has also been shown to reduce the incidence of bladder cancer and significantly improve survival.[81][95][96][97] Their impact on prostate cancer is a bit more complicated. The latest meta-analysis review concluded that five alpha-reductase inhibitors significantly reduced the overall risk of prostate cancer, although a greater incidence of higher-grade prostatic malignancy was found.[81] This did not appear to impact either overall or cancer-specific survival.[98] See the companion StatPearls reference review on "Finasteride" for more information.[99](A1)
Antimuscarinics are commonly used for urinary frequency, urgency, and bladder overactivity symptoms. They are also useful for the symptomatic management of detrusor instability due to bladder outlet obstruction from BPH, which can result in increased urgency (overactive bladder) and frequency. Muscarinic receptor antagonists can help with these symptoms by blocking muscarinic receptors in the detrusor muscle. This reduces smooth muscle tone and can improve irritative symptoms in those with bladder overactivity. Examples include solifenacin, tolterodine, trospium, and oxybutynin.
Those who fail antimuscarinic treatment may be considered for mirabegron or vibegron use (beta-3 adrenoreceptor agonists), which also cause detrusor relaxation and relieve symptoms of overactivity with no cholinergic or mental side effects.
Bladder-relaxing therapies for men with BPH may be used alone if there is good urinary flow and low PVRs. However, they are often used together with alpha-blockers for most patients with symptomatic BPH being treated medically to reduce postvoid volumes, provide symptom relief, and minimize the risk of urinary retention. See the companion StatPearls reference review on "Anticholinergic Medications" for more information.[100]
Surgery
Patients who fail or do not tolerate medical therapy should undergo further evaluation and consider surgical intervention.
Guidelines for the indications for surgery in BPH as outlined by the European Association of Urology (EAU) and the AUA include the following: [57][101]
- Bladder stones
- Hematuria refractory to medical treatment (other causes excluded)
- High-pressure chronic or recurrent urinary retention (absolute indication)
- Intolerance of medical therapy
- Increased postvoid residual (typically >200 mL)
- Recurrent urinary infections
- Refractory urinary retention
- Renal failure with bilateral hydronephrosis due to bladder outlet obstruction
- Renal insufficiency
- Urinary symptoms unresponsive to medical therapy
Surgical management of BPH has broadened significantly over the years, with the development of further minimally invasive techniques. Current recommended procedures include TURP and newer techniques, such as laser vaporization and holmium laser enucleation, which have largely replaced open prostatectomy. Minimally invasive surgical options such as paclitaxel-coated prostatic balloon dilation, prostatic urethral internal lateral suturing (prostatic urethral lift), transurethral microwave thermotherapy, and water vapor or steam infusion therapy are also available and FDA-approved. In rare cases, prostatic artery embolization can be considered.
Additional recommended studies before surgical interventions include a urinalysis and the following:
- Cystoscopy to assess prostatic size and shape, the degree of urinary obstruction, and the condition of the bladder.
- PSA determination as prostate cancer should be excluded before any prostatic surgery.
- Postvoid residual determination can be measured with a bladder scan, dedicated bladder ultrasound, or a quick straight catheterization. Amounts >200 mL are considered pathological.
- Uroflowmetry. The minimal acceptable urinary flow rate is generally about 13 mL/s peak flow. A voided volume ≥150 mL is sufficient for valid measurement.
- A pressure/flow study should be considered in questionable cases.
- Patients should be informed that surgeries are not guaranteed, and additional treatments may be necessary, particularly after minimally invasive procedures.
- Urodynamics is not routinely necessary but may be considered in selected cases, such as:
- Patients with visibly abnormal bladders on cystoscopy or imaging and those with persistently high residual volumes without direct evidence of obstruction may be considered candidates for these extra studies.
- A detrusor muscle that cannot generate at least 30 mL of water pressure is unlikely to be able to empty satisfactorily after surgery without further intervention, such as clean, self-intermittent catheterization.
- It is best to inform patients of this likelihood before any surgical intervention.
Surgical Treatment Options for the Management of BPH:
Transurethral resection of the prostate (TURP) focuses on debulking the prostate to produce an adequate channel for urine to flow. This is achieved using electrical diathermy to produce a high-frequency current that allows tissue cutting and coagulation. An adequate channel can be created by resecting all obstructing prostatic tissue to allow urine to flow freely. Bipolar diathermy has largely replaced monopolar diathermy techniques for TURP, with increased benefits such as resection in saline and a reduced risk of "TUR syndrome." However, bipolar resection may be somewhat slower and requires new instrumentation.[102] The AUA guidelines approve both monopolar and bipolar instrumentation for surgical BPH treatment.[103]
The primary advantage of TURP surgery is that it definitively removes the blocking tissue, there are no size or shape limits (although few surgeons have the skill to safely do a TURP on a prostate >75 grams in size), and it provides substantial tissue for pathological examination. TURP was developed in the early 1940s but is still considered the "gold standard" surgical treatment for BPH. (See our companion StatPearls reference article on "Transurethral Resection of the Prostate."[102]
Transurethral incision of the prostate is similar to a TURP but limited to just creating a channel in the prostatic urethra along with an incision of the bladder neck. It is typically performed on smaller prostates (<30 gm) in patients with high comorbidities. No prostatic tissue is removed, so appropriate prostate cancer screening needs to be done before surgery. If the bladder neck incision is omitted, normal ejaculation is likely to be preserved.[104](B3)
Transurethral electrovaporization of the prostate is also similar to a TURP but uses a roller or vaporization electrode instead of a cutting loop. This vaporizes the prostatic tissue instead of cutting it. It can be performed with monopolar or bipolar instrumentation. The benefit is increased safety and less bleeding, but the process is slower than a TURP, and no tissue is available for pathological analysis.[105] Overall efficacy in properly selected patients is similar to TURP.[106] It has been largely supplanted by laser vaporization, which provides similar results but can be done much faster.(A1)
Transurethral laser vaporization is one of the more popular options for safely removing obstructing prostatic tissue. Instead of an electrical wire loop cutting the prostatic tissue (as in TURP), this technique uses a laser fiber with an angled tip that directs the laser energy laterally. The high energy of the laser vaporizes the tissue. The surgery is done under direct cystoscopic vision. The laser is directed in a sweeping, rotational manner to cover the entire prostate. It has the main advantage of good hemostasis, so it is often preferred for patients on anticoagulants.[107](A1)
Like electrovaporization, no tissue is available at the end of the procedure for pathological examination. Compared to TURP, vaporization is relatively slow and requires specialized equipment that may not be available in every hospital and surgical facility.[108]
Holmium and Thulium laser enucleation of the prostate is a new therapeutic surgical option for BPH, designed for larger prostates not easily managed by other procedures. Previously, only open prostatectomy allowed an enlarged prostatic adenoma to be removed or enucleated off its capsule. This can now be achieved with laser enucleation, referred to as Holmium laser enucleation of the prostate (HoLEP) or Thulium laser enucleation of the prostate (ThuLEP), depending on the laser used.
Energy is transmitted to the tip of the laser fiber, where the irrigation fluid absorbs it. This creates a vaporization bubble that ablates prostatic tissue with minimal tissue penetration. This is then used to enucleate the prostatic transitional zone along the surgical capsule. A separate device then chops up the prostatic tissue into smaller pieces that can be extracted.
Laser enucleation of larger prostates generally results in shorter Foley catheterization times, fewer perioperative bleeding complications, and reduced inpatient hospital days compared to TURP.[109] Meta-analysis has shown significantly improved urinary flow rates, reduced PVRs, and overall symptom improvement comparable to TURP.[110] (A1)
The main advantage of laser enucleation is the ability to manage larger prostates (>75 to 100 gm) without the need for an open procedure. Other benefits include a lower transfusion rate with no increase in complications compared to TURP. However, limitations include the need for specialized equipment and training, making it less readily available.[111] Also, the number of prostates large enough to require this technique is relatively small, so training and gaining substantial experience with the technique can take time.(A1)
Minimally Invasive Procedures
Several minimally invasive surgeries for symptomatic BPH have been developed. They can help minimize the risk of bleeding, anesthesia, and other complications in patients with high-risk comorbidities. They may also be preferred by selected patients desiring a low-risk surgical option or merely to avoid the need for continuing medications. Since no tissue is retrieved for pathological examination, appropriate prostate cancer screening should be done before any of these treatments. For helpful instructional videos on aquablation and many other prostatic surgeries mentioned here, check the AUA Core Instructional Video Library at auanet.org or auau.auanet.org/core. Many of these same films and other instructional videos are available on YouTube for free.
Aquablation (waterjet hydrodissection or ablation) is a minimally invasive robotic-assisted prostatic procedure developed in 2013 and FDA-approved in 2017. It uses a heat-free high-pressure water jet of normal saline to quickly resect and ablate hyperplastic prostatic tissue. Real-time, high-definition ultrasound imaging is used to make a precise surgical map of the prostate. The mapping is done so the bladder neck and veru are preserved. A transurethral robotically controlled probe then uses an unheated, precisely modulated high-pressure water jet to destroy excess prostatic tissue by automatically following the prepared surgically mapped outline. There is no radiation exposure, no invisible thermal damage, and no harm to immediately adjacent tissue, as only the center of the waterjet stream is sufficiently robust to cause tissue ablation. The aquablation procedure is performed as follows: [112][113](B3)
A transrectal ultrasound probe is inserted into the rectum and adjusted for good visualization of the prostate both sagitally and basically. It is then placed in a special cradle mounting for stability. The 24 French cystoscopic probe is then guided into the bladder, secured to the robotic actuating arm, and carefully aligned with the live ultrasound image. The cystoscope is placed just distal to the very with the waterjet probe advanced to the bladder neck or median lobe as necessary. The prostate is mapped and outlined on the ultrasound image, and strategic anatomical locations (median lobe, bladder neck, mid prostate, veru) are carefully marked. Automatic computer-modulated variations in water pressure precisely control the depth of water jet penetration up to a maximum of 25 mm as the water jet exits the probe at a 90º angle.
The probe is robotically rotated laterally (right and left) up to the preset limits, with a maximum of 225º, to engage the obstructing tissue as determined by the surgical map settings. It is then slowly and continuously withdrawn automatically, starting at the median lobe or bladder neck, and progressing close to or up to the veru to cover the entire prostate, except anteriorly, where the tissue is not treated. The ablative treatment process takes only a few minutes and is continuously monitored by real-time ultrasonography and cystoscopic imaging. Most patients require a second pass (about 90%), and some may need more.
An immediate examination with a resectoscope is then performed to inspect the prostatic fossa and provide hemostasis. Aquablation does not coagulate, so bleeding must be controlled by transurethral electrocauterization with a resectoscope using a standard loop or rollerball. Transurethral resection of remaining prostatic tissue may sometimes be needed to minimize obstruction or to better visualize the bleeding points for cauterization. Particular attention should be given to bleeding sites at the bladder neck that may need focal cauterization.
After achieving hemostasis, the resectoscope is removed, and a 3-way Foley catheter with continuous irrigation is placed, completing the procedure. Some practitioners may prefer to use traction.[112] The catheter can often be removed the following day. The actual aquablation therapy time is only 5 to 10 minutes, and the entire procedure takes less than an hour. However, it requires anesthesia, and many patients will need an overnight hospital stay.[114]
The optimal prostate size for the procedure is from 30 to 80 grams, but aquablation can be used in glands of any size.[115] Unlike most other minimally invasive procedures, aquablation can be used in patients with large median lobes and prostates up to 150 grams or more.[116][117][118] It may even provide superior results in these large and extra-large prostate glands compared to TURP.[119] (A1)
The advantages of the aquablation procedure include a postoperative TURP defect with very low rates of incontinence, retrograde ejaculation, or erectile dysfunction compared to other surgeries.[120] It has also improved the rate of incontinence and bladder overactivity in patients who had such symptoms preoperatively and demonstrated a surgical retreatment rate of only 0.7%.[120] It can be used even in very large prostates and for large median lobes. Outcome measurements appear durable and comparable to TURP after five years, with efficacy and safety superior to TURP for prostates greater than 50 grams in size.[118](A1)
The main disadvantages of aquablation therapy are the very high cost of the equipment needed to perform the procedure and the lack of any significant prostatic tissue retrieved afterward for histological examination. This means that prostate biopsies should be performed preoperatively in suspicious cases before treatment. Side effects are relatively mild, including temporary dysuria, hematuria, pelvic discomfort, urinary retention, urgency, frequency, and urinary tract infections. It is not suitable for patients on active anticoagulation or who have uncorrected coagulopathies.
Aquablation provides superior long-term relief of symptoms compared to standard transurethral resection (TURP) for prostates between 50 and 80 ccs in volume with fewer complications, much less ejaculatory dysfunction, and a significantly lower rate of erectile dysfunction (ED).[121][122] Multiple studies have reported excellent results with aquablation, equal or even superior to transurethral resection of the prostate, with fewer complications, shorter operating times, and a reduced incidence of postoperative ejaculatory disorders, erectile dysfunction, or incontinence.[114][115][118][119][121][122][121][123][124][125][126][127][128][129](A1)
Aquablation Tips:
- When treating the median lobe, care should be taken to avoid injury to the trigone, which may be immediately below. Increasing the bladder volume can help increase the distance between the two structures.
- Avoid slicing the median lobe off completely, as this would result in a sizeable prostatic tissue mass in the bladder.
- Maximum water pressure can be reduced when dealing with smaller prostates. These smaller prostates can be challenging since the anatomical structures are so close together.
Paclitaxel-coated prostatic balloon dilation is now FDA-approved as a minimally invasive therapy for BPH. Paclitaxel is a taxane-based chemotherapy drug used to treat breast, ovarian, and non-small cell lung cancer, as well as Kaposi's sarcoma, lymphoma, and leukemia.[130] It's a microtubule-stabilizing compound that induces mitotic arrest, leading to cellular death.[131] It is also used as the key medication in drug-eluding vascular stents and angioplasty balloons to prevent arterial restenosis, as it has significant anti-proliferative and anti-inflammatory properties.[132][133][134][135][136] (B3)
A prostatic dilating balloon is used to produce an anterior commissurotomy that separates the lateral lobes of the prostate while simultaneously delivering paclitaxel to the prostatic urethra. A prostatic dilation balloon coated with paclitaxel provides uniform, concentric drug delivery to the surrounding prostatic urethral tissue when the balloon is inflated.[137] The paclitaxel prevents new tissue growth and promotes healing with an open urinary passage through the prostatic urethra, allowing normal voiding.[137][138]
Previous balloon dilation treatments of the prostate did not maintain efficacy, but studies indicate that the paclitaxel-coated prostatic balloon dilation treatment retains symptomatic BPH improvement for at least one and as long as four years.[137][138][139][140][141][142] The mean International Prostate Symptoms Score (IPSS) score dropped from a pretreatment baseline of 22.3 to 11.5 at four years, indicating sustained, long-term symptomatic improvement.[138](A1)
The average peak urinary flow rate improved by 119%, from 8.9 mL/s before treatment to 19 mL/s measured a year after the procedure.[137] Advantages of paclitaxel-coated balloon dilation therapy include no reported ED or change in ejaculation, and the symptomatic benefits are noticeable within days of the procedure.[137] Also, the procedure does not involve prostatic incisions or resection, thermal ablation, microwave therapy, tissue burning, vaporization, cutting, steam injection, radiation, or implants.
Prostatic urethra internal lateral suturing (prostatic urethral lift) involves compressing the coapting and obstructing prostate lobes laterally. This allows the urinary channel to be substantially widened without cutting or resectioning the prostate while leaving the bladder neck intact. This preserves ejaculation and sexual function while still improving LUTS. Studies have shown preserved sexual function, improved symptom scores (IPSS, AUA), and higher peak urinary flow rates (QMax), but proper patient selection is important.[143] Peak flow rates typically improve by 30%.
Improvement in urinary symptoms occurs very quickly, typically within a few days to weeks. The procedure is suitable for patients with prostate sizes 30 cc to 80 cc. It is unsuitable for patients with a significantly enlarged median lobe, especially if there is a large intravesical component, as the treatment is performed only on the obstructing lateral lobes. Transurethral resection, electrovaporization, or laser vaporization of the median lobe can be performed, but that changes the procedure's minimally invasive nature. The overall success rate is about 85%.[144] For more information, see the companion StatPearls reference review on "Prostatic Urethral Lift."[144]
Water vapor thermal infusion is primarily an office-based procedure designed for prostates 30 cc to 80 cc in size. Minimal anesthesia is required, making it suitable for frail patients unable to tolerate a full anesthetic.[145] Steam is injected directly into the prostate under cystoscopic guidance during the procedure through a retractable transurethral needle in 9-second bursts. A microwave generator produces the steam. Unlike direct microwave therapy, there is limited tissue penetration as the heat transfer is convection-controlled and very localized. Peak flow rates improved by 6 mL/sec.[146] Sexual function and ejaculation were unaffected since the bladder neck area was untreated.[147] (A1)
The treatment diminishes the prostate size by about 30% over time, and this reduction is localized to the obstructing lobes[148]. Unlike prostatic lift procedures, water vapor thermal infusion therapy can also treat median lobe enlargement. Overall, it is considered safe and effective for most patients with BPH.[149][150] Efficacy is similar to prostatic urethral lift procedures, but water vapor infusion is considerably less costly.[151] However, it takes longer to perform and requires more time until peak efficacy is achieved, typically about three months. (A1)
A recent study found good efficacy in selected patients with prostates larger than 80 mL.[152] In this study, the IPSS score improved by 70%, and peak urinary flow increased by 59% after 12 months without adversely affecting sexual performance.[152] For more information, see the companion StatPearls reference review on "Water Vapor Thermal Ablation of the Prostate."[153]
Transurethral microwave thermotherapy is an outpatient procedure to treat BPH that uses a microwave antenna inserted transurethrally into the prostatic urethra to treat obstructing tissue. This causes heat necrosis of the obstructive tissue, which is then slowly reabsorbed over time, resulting in an open prostatic passage and normalized voiding. It is generally considered a safe procedure with relatively few side effects but is done blindly without direct cystoscopic observation.[154] Transurethral microwave thermotherapy (TUMT) requires specialized equipment, and results have sometimes been spotty with relatively high retreatment rates.[154] It is most suitable for men with significant comorbidities, making more invasive surgical options unacceptable.
Selective prostatic artery embolization can be effective for controlling otherwise intractable prostatic bleeding. Incidentally, it has also been found to shrink prostatic enlargement from BPH. The procedure is technically demanding and requires a skilled interventional radiologist to perform. Efficacy appears reasonable, if not quite equivalent to standard BPH surgery. It is intended mainly for patients who are too frail or ill for other surgical procedures.[155][156][157][158][159] Prostatic artery embolization for BPH is still considered investigational.[57](A1)
Experimental Treatments and Other Procedures No Longer Available or Recommended:
- The 2021 AUA Guidelines on Management of BPH/LUTS no longer recommend transurethral needle ablation (TUNA) due to its unpredictable and unreliable results.
- Indigo laser prostatic thermotherapy, which uses a diffusing laser fiber inserted directly into the obstructing prostatic lobes under direct vision via cystoscopy, is no longer available. While effective, considerable operator skill and experience were needed for optimal results. Also, the unique laser fibers required had limited built-in treatment activations and were quite costly.
- Prostatic balloon dilation using a spherical balloon is another minimally invasive procedure that initially produced promising results, which has also now been discredited and is no longer used or recommended.[141][142] The treatment was found to primarily rupture the prostatic capsule rather than permanently treat the tissue of the obstructing lobes. When the ruptured capsule healed, symptoms returned as the prostate grew.
- Recently, the use of a columnar balloon for prostatic dilation has been investigated, and early results appear promising.[160][161][162]
- High-intensity focused ultrasound can reliably ablate prostatic tissue. Like water vapor thermal infusion, it takes some time to be fully effective and for the ablated tissue to be reabsorbed. It is still considered investigational at this time. (A1)
Differential Diagnosis
Differential diagnoses for LUTS are broad and include the following:
- Atonic or decompensated bladders
- Bladder neck strictures
- Bladder or prostate cancer
- Bladder overactivity
- Cauda equina syndrome or neurogenic bladder (can present with acute retention)
- High-pressure chronic retention (presentation can be insidious or with acute renal failure)
- Hydronephrosis
- Neurogenic bladder (can be secondary to Parkinson's, multiple sclerosis, diabetes, etc)
- Penile cancer
- Phimosis
- Prostate cancer
- Prostatitis
- Renal failure
- Sexually transmitted diseases
- Spinal disorders
- Urethral strictures
- Urinary retention
- UTIs
- Urinary tract stones (bladder stones)
Prognosis
Deterioration in LUTS with increasingly problematic voiding symptoms is the most common indicator of BPH disease progression. Patients may also present with complications, including urinary retention, infections, or hematuria.
Observational studies have demonstrated that when left without treatment, clinical progression of BPH increased over 48 months, with 31% of the cohort requiring further treatment and 5% developing acute retention in the same period.[75]
The risk of acute urinary retention increases with age. In an Olmsted County, Minnesota study, the incidence of retention in men increased over 10-fold, from 3 per 1000 (40 to 49 years) to 34.7 per 1000 (70 to 79 years).[163] Left untreated, BPH has a significant risk of progression. Men with significantly enlarged prostates (>30 gm) are at increased risk of disease progression.[164] In another study, up to 42% of men who presented with urinary retention went on to have surgery.[165]
The 5 alpha-reductase inhibitors have been shown to reduce the incidence of urinary retention and delay the need for surgery, while alpha-blockers have not.
Complications
Common Complications
- Acute urinary retention
- Bladder calculi
- Chronic urinary retention
- Decompensated bladder
- Detrusor hypotonicity
- Elevated PSA unrelated to prostate cancer
- Hematuria
- Hydronephrosis
- Incomplete bladder emptying
- Postoperative incontinence
- Renal failure
- Suprapubic distension or discomfort
- UTIs (due to incomplete emptying)
- Weak or intermittent urinary stream
Other complications may arise as a result of catheterization for the management of LUTS in BPH and include the following:
- Failed trial without catheter
- Long-term catheter complications (blocked catheters, retention, hematuria, UTIs)
Hematuria
This complication is common in BPH patients and a frequent cause for a urological referral for further investigation. Due to the increased vascularity of larger prostates, small superficial vessels may be disrupted, causing bleeding.[71] Finasteride has been shown to decrease the density of vessels and can help manage problematic BPH-related hematuria.[166]
Postoperative Incontinence
A serious complication of BPH surgery is incontinence. A small amount of leakage for a short time after surgery is common after many procedures, but severe, chronic urinary leakage would be quite disturbing. Fortunately, this is a rare complication, particularly after one of the newer, minimally invasive procedures.
Treatments will vary somewhat depending on which surgery was performed and the severity of the leakage. Initial therapies include pads, penile clamps, condom catheters, Kegel exercises, anticholinergics, and physical therapy.[167] American Urological Association guidelines recommend male slings and adjustable implantable urethral compression balloons in selected patients with mild-to-moderate stress incontinence who do not respond to more conservative treatments.[167] Artificial urinary sphincters are recommended in the most severe cases of postoperative male stress incontinence, although adjustable urethral compression balloons may also be used in nonradiated patients.[167][168] For more details, see the companion StatPearls' reference on "Artificial Urinary Sphincters and Adjustable Dual-Balloon Continence Therapy in Men."[168]
Urinary Tract Infections
Urinary tract infection complications occur due to incomplete bladder emptying, resulting in stagnant urine, which promotes infections and stone formation. Recurrent infections may indicate a need for additional treatment to prevent associated comorbidities and complications.
Deterrence and Patient Education
Lifestyle factors such as weight loss and improved diabetic control should be explained to the patient to allow modifiable risk factors to be addressed. This may also help reduce the risk of future BPH surgery. Lifestyle measures such as reducing caffeine and optimizing the timing of fluid intake can also be used to address specific problematic urinary symptoms. For example, if the patient takes furosemide, administering the medication 6 to 8 hours before bedtime helps reduce nocturia.[50]
Patients managed with long-term catheters or intermittent self-catheterization should be taught the importance of hygiene and catheter care to prevent UTIs. This may be done with the assistance of dedicated specialist nurses. The provision for managing the catheter in the community should also be made.
Patients with BPH should understand the risks of disease progression before committing to treatment options and should be counseled on alternative management, such as watchful waiting, medical therapy, and any other surgeries available to make an informed decision.
Pearls and Other Issues
Key thoughts to consider regarding BHP:
- BPH is evaluated using a urinalysis, a PSA test, a DRE, a symptom score (IPSS or AUA), and a PVR.
- A 24-hour voiding diary can be helpful if the patient has significant frequency or nocturia.
- Asymmetry on a DRE should be considered possibly suspicious for prostate cancer.
- Bladder scanners are extremely useful in evaluating BPH, abdominal pain, suprapubic fullness, incontinence, and various voiding disorders, even in primary care clinics.
- Noninvasive testing (bladder scan for postvoid residual determinations and flowmetry), along with a PSA test and DRE, are usually sufficient to diagnose and treat symptomatic BPH medically.
- A bladder scan is recommended for all primary care offices to allow for noninvasive testing of postvoid residual urine volumes in all males older than 50 years with urinary symptoms.
- A renal ultrasound is strongly recommended in patients with BPH and unexplained renal failure or urinary retention.
- Persistent hydronephrosis after adequate urinary drainage with a catheter suggests possible prostate cancer.
- When treating patients with alpha-blockers for acute retention, use a maximum dose and allow at least 72 hours before any voiding trial. (The dosage can always be reduced later.)
- Medications for BPH treatment typically require at least 4 weeks or longer for evaluation. Five alpha-reductase inhibitors require 6 months to achieve full effectiveness.
- Five alpha-reductase medications can be used to reduce prostatic bleeding regardless of any surgery.
- Before using anticholinergics or beta-agonists for overactive bladder symptoms, ensure that the urinary flow rate and PVR are acceptable.
- Patients choosing less invasive therapies should be informed that such treatments often have less symptomatic improvement than more aggressive surgeries.
- Although generally considered investigational, prostatic artery embolization can be performed for carefully selected patients, typically individuals who are too ill or frail for other procedures, if the technical expertise is available.
- Always ask your emergency room department to record the volume drained immediately after placing a Foley catheter for urinary retention, as this helps predict the detrusor muscle's rehabilitation time.
- Do not clamp the Foley catheter or restrict drainage when a residual urine volume of 1500 mL or more is drained. Allow it to drain freely. Be aware of the possibility of post-obstructive diuresis.
- If no option is available other than long-term catheter drainage, a suprapubic tube is generally preferred over a urethral Foley for patient comfort and reduced complications.
Enhancing Healthcare Team Outcomes
Addressing BPH requires a multifaceted approach from physicians, advanced practitioners, nurses, pharmacists, physical therapists, and other health professionals. Clinicians caring for BPH patients must possess diagnostic skills to differentiate the severity of the condition and determine appropriate interventions. Developing a comprehensive strategy involves evidence-based decision-making, considering individual patient preferences. Professionals need strategic thinking to address BPH's varied clinical presentations, ensuring a tailored approach that aligns with patient needs and overall well-being. Competence in selecting and implementing medical or surgical treatments based on patient factors is crucial.
Medical outcomes can be optimized through the use of IPSS or AUA scoring systems. These can help stratify patients according to symptom severity and guide decision-making. Adherence to lifestyle factors affecting BPH may also be addressed through dietary advice, weight loss, exercise, and improved glycemic control. Endocrinologists, nutritionists, and diabetic nurse specialists can help address these areas, and early referral to such experts is recommended. Optimizing these factors before surgery can also be beneficial in reducing the risk of comorbidities and postoperative complications.
Catheter care is important for patients performing intermittent self-catheterization due to symptoms or with long-term Foleys. This can be addressed by specialist nurses who can help educate the patient to ensure adequate training, support, and follow-up in the community. In the UK, the Department of Health advocates integrating continence services for those with long-term urinary problems.[169] Those involved in community catheter care and management should be aware of the indications for catheterization and hospital referrals; implementing a catheter passport can help with this.[170]
Pharmacists are particularly important due to the increasing incidence of BPH with age and the high rate of polypharmacy associated with geriatric patients. It is critically important that any drug interactions are promptly and properly identified. The use of concomitant anticoagulants should be recognized to ensure that they may be appropriately held before surgery or other invasive procedures offered to the patient.
Overall, close interplay is needed between primary care physicians, advanced care practitioners, urologists, nephrologists, nurses, pharmacists, and physical therapists to ensure patients are managed and referred appropriately. By honing skills, employing strategic decision-making, sharing responsibilities, fostering communication, and coordinating care effectively, the interprofessional team can contribute to patient-centered care, improved outcomes, patient safety, and enhanced team performance in BPH management.
Media
(Click Image to Enlarge)
References
Roehrborn CG. Benign prostatic hyperplasia: an overview. Reviews in urology. 2005:7 Suppl 9(Suppl 9):S3-S14 [PubMed PMID: 16985902]
Level 3 (low-level) evidenceAbrams P. LUTS, BPH, BPE, BPO: A Plea for the Logical Use of Correct Terms. Reviews in urology. 1999 Spring:1(2):65 [PubMed PMID: 16985774]
Silverman WM. "Alphabet soup" and the prostate: LUTS, BPH, BPE, and BOO. The Journal of the American Osteopathic Association. 2004 Feb:104(2 Suppl 2):S1-4 [PubMed PMID: 15038396]
Level 3 (low-level) evidenceAbrams P. New words for old: lower urinary tract symptoms for "prostatism". BMJ (Clinical research ed.). 1994 Apr 9:308(6934):929-30 [PubMed PMID: 8173393]
Roehrborn CG. Pathology of benign prostatic hyperplasia. International journal of impotence research. 2008 Dec:20 Suppl 3():S11-8. doi: 10.1038/ijir.2008.55. Epub [PubMed PMID: 19002119]
Level 3 (low-level) evidenceParsons JK. Benign Prostatic Hyperplasia and Male Lower Urinary Tract Symptoms: Epidemiology and Risk Factors. Current bladder dysfunction reports. 2010 Dec:5(4):212-218 [PubMed PMID: 21475707]
Chughtai B, Forde JC, Thomas DD, Laor L, Hossack T, Woo HH, Te AE, Kaplan SA. Benign prostatic hyperplasia. Nature reviews. Disease primers. 2016 May 5:2():16031. doi: 10.1038/nrdp.2016.31. Epub 2016 May 5 [PubMed PMID: 27147135]
Schenk JM, Calip GS, Tangen CM, Goodman P, Parsons JK, Thompson IM, Kristal AR. Indications for and use of nonsteroidal antiinflammatory drugs and the risk of incident, symptomatic benign prostatic hyperplasia: results from the prostate cancer prevention trial. American journal of epidemiology. 2012 Jul 15:176(2):156-63. doi: 10.1093/aje/kwr524. Epub 2012 Jun 28 [PubMed PMID: 22759721]
Level 2 (mid-level) evidenceSutcliffe S, Grubb Iii RL, Platz EA, Ragard LR, Riley TL, Kazin SS, Hayes RB, Hsing AW, Andriole GL, Urologic Diseases in America Project. Non-steroidal anti-inflammatory drug use and the risk of benign prostatic hyperplasia-related outcomes and nocturia in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. BJU international. 2012 Oct:110(7):1050-9. doi: 10.1111/j.1464-410X.2011.10867.x. Epub 2012 Mar 19 [PubMed PMID: 22429766]
Level 1 (high-level) evidenceNygård LH, Talala K, Taari K, Tammela TLJ, Auvinen A, Murtola TJ. The effect of non-steroidal anti-inflammatory drugs on risk of benign prostatic hyperplasia. The Prostate. 2017 Jun:77(9):1029-1035. doi: 10.1002/pros.23359. Epub [PubMed PMID: 28480542]
Kukko V, Kaipia A, Talala K, Taari K, Tammela TLJ, Auvinen A, Murtola TJ. Allopurinol and risk of benign prostatic hyperplasia in a Finnish population-based cohort. Prostate cancer and prostatic diseases. 2018 Sep:21(3):373-378. doi: 10.1038/s41391-017-0031-8. Epub 2017 Dec 22 [PubMed PMID: 29273728]
Foster CS. Pathology of benign prostatic hyperplasia. The Prostate. Supplement. 2000:9():4-14 [PubMed PMID: 11056496]
Isaacs JT. Antagonistic effect of androgen on prostatic cell death. The Prostate. 1984:5(5):545-57 [PubMed PMID: 6483690]
Level 3 (low-level) evidenceGann PH, Hennekens CH, Longcope C, Verhoek-Oftedahl W, Grodstein F, Stampfer MJ. A prospective study of plasma hormone levels, nonhormonal factors, and development of benign prostatic hyperplasia. The Prostate. 1995 Jan:26(1):40-9 [PubMed PMID: 7531326]
Level 2 (mid-level) evidenceRohrmann S, Smit E, Giovannucci E, Platz EA. Association between markers of the metabolic syndrome and lower urinary tract symptoms in the Third National Health and Nutrition Examination Survey (NHANES III). International journal of obesity (2005). 2005 Mar:29(3):310-6 [PubMed PMID: 15672112]
Level 2 (mid-level) evidenceNygård LH, Talala K, Taari K, Tammela TLJ, Auvinen A, Murtola TJ. Antidiabetic drugs, glycemic control and risk of benign prostatic hyperplasia. The Prostate. 2023 Feb:83(3):246-258. doi: 10.1002/pros.24456. Epub 2022 Nov 3 [PubMed PMID: 36325820]
Maserejian NN, Wager CG, Giovannucci EL, Curto TM, McVary KT, McKinlay JB. Intake of caffeinated, carbonated, or citrus beverage types and development of lower urinary tract symptoms in men and women. American journal of epidemiology. 2013 Jun 15:177(12):1399-410. doi: 10.1093/aje/kws411. Epub 2013 May 30 [PubMed PMID: 23722012]
Crispo A, Talamini R, Gallus S, Negri E, Gallo A, Bosetti C, La Vecchia C, Dal Maso L, Montella M. Alcohol and the risk of prostate cancer and benign prostatic hyperplasia. Urology. 2004 Oct:64(4):717-22 [PubMed PMID: 15491708]
Level 2 (mid-level) evidenceMaserejian NN, Giovannucci EL, McVary KT, McKinlay JB. Dietary, but not supplemental, intakes of carotenoids and vitamin C are associated with decreased odds of lower urinary tract symptoms in men. The Journal of nutrition. 2011 Feb:141(2):267-73. doi: 10.3945/jn.110.132514. Epub 2010 Dec 22 [PubMed PMID: 21178086]
Level 2 (mid-level) evidenceSanda MG, Beaty TH, Stutzman RE, Childs B, Walsh PC. Genetic susceptibility of benign prostatic hyperplasia. The Journal of urology. 1994 Jul:152(1):115-9 [PubMed PMID: 7515446]
Level 2 (mid-level) evidenceMeikle AW, Bansal A, Murray DK, Stephenson RA, Middleton RG. Heritability of the symptoms of benign prostatic hyperplasia and the roles of age and zonal prostate volumes in twins. Urology. 1999 Apr:53(4):701-6 [PubMed PMID: 10197844]
Rohrmann S, Fallin MD, Page WF, Reed T, Partin AW, Walsh PC, Platz EA. Concordance rates and modifiable risk factors for lower urinary tract symptoms in twins. Epidemiology (Cambridge, Mass.). 2006 Jul:17(4):419-27 [PubMed PMID: 16699472]
Lloyd GL, Ricke WA, McVary KT. Inflammation, Voiding and Benign Prostatic Hyperplasia Progression. The Journal of urology. 2019 May:201(5):868-870. doi: 10.1097/JU.0000000000000049. Epub [PubMed PMID: 30694937]
Robert G, Descazeaud A, Nicolaïew N, Terry S, Sirab N, Vacherot F, Maillé P, Allory Y, de la Taille A. Inflammation in benign prostatic hyperplasia: a 282 patients' immunohistochemical analysis. The Prostate. 2009 Dec 1:69(16):1774-80. doi: 10.1002/pros.21027. Epub [PubMed PMID: 19670242]
Level 2 (mid-level) evidenceLi J, Li Y, Cao D, Huang Y, Peng L, Meng C, Wei Q. The association between histological prostatitis and benign prostatic hyperplasia: a single-center retrospective study. The aging male : the official journal of the International Society for the Study of the Aging Male. 2022 Dec:25(1):88-93. doi: 10.1080/13685538.2022.2050360. Epub [PubMed PMID: 35289705]
Level 2 (mid-level) evidenceKahokehr A, Vather R, Nixon A, Hill AG. Non-steroidal anti-inflammatory drugs for lower urinary tract symptoms in benign prostatic hyperplasia: systematic review and meta-analysis of randomized controlled trials. BJU international. 2013 Feb:111(2):304-11. doi: 10.1111/j.1464-410X.2012.11559.x. Epub [PubMed PMID: 23356748]
Level 1 (high-level) evidenceKristal AR, Arnold KB, Schenk JM, Neuhouser ML, Weiss N, Goodman P, Antvelink CM, Penson DF, Thompson IM. Race/ethnicity, obesity, health related behaviors and the risk of symptomatic benign prostatic hyperplasia: results from the prostate cancer prevention trial. The Journal of urology. 2007 Apr:177(4):1395-400; quiz 1591 [PubMed PMID: 17382740]
Parsons JK, Carter HB, Partin AW, Windham BG, Metter EJ, Ferrucci L, Landis P, Platz EA. Metabolic factors associated with benign prostatic hyperplasia. The Journal of clinical endocrinology and metabolism. 2006 Jul:91(7):2562-8 [PubMed PMID: 16608892]
Level 2 (mid-level) evidenceFurukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. The Journal of clinical investigation. 2004 Dec:114(12):1752-61 [PubMed PMID: 15599400]
Level 3 (low-level) evidenceDe Nunzio C, Aronson W, Freedland SJ, Giovannucci E, Parsons JK. The correlation between metabolic syndrome and prostatic diseases. European urology. 2012 Mar:61(3):560-70. doi: 10.1016/j.eururo.2011.11.013. Epub 2011 Nov 15 [PubMed PMID: 22119157]
Gacci M, Corona G, Vignozzi L, Salvi M, Serni S, De Nunzio C, Tubaro A, Oelke M, Carini M, Maggi M. Metabolic syndrome and benign prostatic enlargement: a systematic review and meta-analysis. BJU international. 2015 Jan:115(1):24-31. doi: 10.1111/bju.12728. Epub 2014 Aug 16 [PubMed PMID: 24602293]
Level 1 (high-level) evidenceGolbidi S, Laher I. Bladder dysfunction in diabetes mellitus. Frontiers in pharmacology. 2010:1():136. doi: 10.3389/fphar.2010.00136. Epub 2010 Nov 16 [PubMed PMID: 21833175]
Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. The Journal of urology. 1984 Sep:132(3):474-9 [PubMed PMID: 6206240]
Platz EA, Joshu CE, Mondul AM, Peskoe SB, Willett WC, Giovannucci E. Incidence and progression of lower urinary tract symptoms in a large prospective cohort of United States men. The Journal of urology. 2012 Aug:188(2):496-501. doi: 10.1016/j.juro.2012.03.125. Epub 2012 Jun 15 [PubMed PMID: 22704110]
Level 2 (mid-level) evidenceLoeb S, Kettermann A, Carter HB, Ferrucci L, Metter EJ, Walsh PC. Prostate volume changes over time: results from the Baltimore Longitudinal Study of Aging. The Journal of urology. 2009 Oct:182(4):1458-62. doi: 10.1016/j.juro.2009.06.047. Epub 2009 Aug 15 [PubMed PMID: 19683305]
Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in America project: benign prostatic hyperplasia. The Journal of urology. 2005 Apr:173(4):1256-61 [PubMed PMID: 15758764]
Kupelian V, Wei JT, O'Leary MP, Kusek JW, Litman HJ, Link CL, McKinlay JB, BACH Survery Investigators. Prevalence of lower urinary tract symptoms and effect on quality of life in a racially and ethnically diverse random sample: the Boston Area Community Health (BACH) Survey. Archives of internal medicine. 2006 Nov 27:166(21):2381-7 [PubMed PMID: 17130393]
Level 2 (mid-level) evidenceParsons JK, Bergstrom J, Silberstein J, Barrett-Connor E. Prevalence and characteristics of lower urinary tract symptoms in men aged } or = 80 years. Urology. 2008 Aug:72(2):318-21. doi: 10.1016/j.urology.2008.03.057. Epub 2008 Jun 12 [PubMed PMID: 18554695]
Stroup SP, Palazzi-Churas K, Kopp RP, Parsons JK. Trends in adverse events of benign prostatic hyperplasia (BPH) in the USA, 1998 to 2008. BJU international. 2012 Jan:109(1):84-7. doi: 10.1111/j.1464-410X.2011.10250.x. Epub 2011 May 26 [PubMed PMID: 21615853]
Level 2 (mid-level) evidenceCenters for Disease Control and Prevention (CDC). Trends in aging--United States and worldwide. MMWR. Morbidity and mortality weekly report. 2003 Feb 14:52(6):101-4, 106 [PubMed PMID: 12645839]
Jin B, Turner L, Zhou Z, Zhou EL, Handelsman DJ. Ethnicity and migration as determinants of human prostate size. The Journal of clinical endocrinology and metabolism. 1999 Oct:84(10):3613-9 [PubMed PMID: 10523004]
Ganpule AP, Desai MR, Desai MM, Wani KD, Bapat SD. Natural history of lower urinary tract symptoms: preliminary report from a community-based Indian study. BJU international. 2004 Aug:94(3):332-4 [PubMed PMID: 15291862]
Caine M. The present role of alpha-adrenergic blockers in the treatment of benign prostatic hypertrophy. The Journal of urology. 1986 Jul:136(1):1-4 [PubMed PMID: 2423716]
Foo KT. Pathophysiology of clinical benign prostatic hyperplasia. Asian journal of urology. 2017 Jul:4(3):152-157. doi: 10.1016/j.ajur.2017.06.003. Epub 2017 Jun 13 [PubMed PMID: 29264224]
Lepor H. Pathophysiology of benign prostatic hyperplasia in the aging male population. Reviews in urology. 2005:7 Suppl 4(Suppl 4):S3-S12 [PubMed PMID: 16986052]
Babinski MA, Manaia JH, Cardoso GP, Costa WS, Sampaio FJ. Significant decrease of extracellular matrix in prostatic urethra of patients with benign prostatic hyperplasia. Histology and histopathology. 2014 Jan:29(1):57-63. doi: 10.14670/HH-29.57. Epub 2013 Jun 21 [PubMed PMID: 23788026]
Schenk JM, Kristal AR, Arnold KB, Tangen CM, Neuhouser ML, Lin DW, White E, Thompson IM. Association of symptomatic benign prostatic hyperplasia and prostate cancer: results from the prostate cancer prevention trial. American journal of epidemiology. 2011 Jun 15:173(12):1419-28. doi: 10.1093/aje/kwq493. Epub 2011 May 3 [PubMed PMID: 21540324]
Level 2 (mid-level) evidenceMcNeal J. Pathology of benign prostatic hyperplasia. Insight into etiology. The Urologic clinics of North America. 1990 Aug:17(3):477-86 [PubMed PMID: 1695776]
Level 3 (low-level) evidenceHoshiyama F, Hirayama A, Tanaka M, Taniguchi M, Ohi M, Momose H, Nakamura T, Ogawa S, Torimoto K, Tanaka N, Fujimoto K. The impact of obstructive sleep apnea syndrome on nocturnal urine production in older men with nocturia. Urology. 2014 Oct:84(4):892-6. doi: 10.1016/j.urology.2014.02.073. Epub 2014 Aug 2 [PubMed PMID: 25096335]
Leslie SW, Sajjad H, Singh S. Nocturia. StatPearls. 2024 Jan:(): [PubMed PMID: 30085529]
Chang TL, Chen SF, Kuo HC. Surgical outcome of male patients with chronic central nervous system disorders and voiding dysfunction due to bladder outlet obstruction. International urology and nephrology. 2022 Oct:54(10):2511-2519. doi: 10.1007/s11255-022-03285-3. Epub 2022 Jul 11 [PubMed PMID: 35821368]
Li FF, Cui YS, Yan R, Cao SS, Feng T. Prevalence of lower urinary tract symptoms, urinary incontinence and retention in Parkinson's disease: A systematic review and meta-analysis. Frontiers in aging neuroscience. 2022:14():977572. doi: 10.3389/fnagi.2022.977572. Epub 2022 Sep 12 [PubMed PMID: 36172485]
Level 1 (high-level) evidenceZiadeh T, Mjaess G, El Helou J, Zalaket J, Mouawad C, Azar C, Abboud H, Koussa S, Nemr E, El Helou E. Impact on quality of life in multiple sclerosis patients: Which urinary symptoms are to blame? Progres en urologie : journal de l'Association francaise d'urologie et de la Societe francaise d'urologie. 2022 Sep:32(10):711-716. doi: 10.1016/j.purol.2022.05.003. Epub 2022 Jun 14 [PubMed PMID: 35715252]
Level 2 (mid-level) evidenceQasrawi H, Tabouni M, Almansour SW, Ghannam M, Abdalhaq A, Abushamma F, Koni AA, Zyoud SH. An evaluation of lower urinary tract symptoms in diabetic patients: a cross-sectional study. BMC urology. 2022 Nov 10:22(1):178. doi: 10.1186/s12894-022-01133-1. Epub 2022 Nov 10 [PubMed PMID: 36357918]
Level 2 (mid-level) evidenceFisch GZ, Fang AH, Miller CD, Choi C, Monaghan TF, Smith EF, Prishtina L, Weiss JP, Blaivas JG. Polyuria in patients with lower urinary tract symptoms: Prevalence and etiology. Neurourology and urodynamics. 2023 Jan:42(1):256-262. doi: 10.1002/nau.25078. Epub 2022 Nov 1 [PubMed PMID: 36317410]
Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, Gubbi S, Hannah-Shmouni F, Koch CA, Verbalis JG. Diagnostic Testing for Diabetes Insipidus. Endotext. 2000:(): [PubMed PMID: 30779536]
Lerner LB, McVary KT, Barry MJ, Bixler BR, Dahm P, Das AK, Gandhi MC, Kaplan SA, Kohler TS, Martin L, Parsons JK, Roehrborn CG, Stoffel JT, Welliver C, Wilt TJ. Management of Lower Urinary Tract Symptoms Attributed to Benign Prostatic Hyperplasia: AUA GUIDELINE PART I-Initial Work-up and Medical Management. The Journal of urology. 2021 Oct:206(4):806-817. doi: 10.1097/JU.0000000000002183. Epub 2021 Aug 13 [PubMed PMID: 34384237]
Jain MA, Leslie SW, Sapra A. Prostate Cancer Screening. StatPearls. 2024 Jan:(): [PubMed PMID: 32310541]
Barry MJ, Fowler FJ Jr, O'leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, Cockett AT, Measurement Committee of the American Urological Association. The American Urological Association Symptom Index for Benign Prostatic Hyperplasia. The Journal of urology. 2017 Feb:197(2S):S189-S197. doi: 10.1016/j.juro.2016.10.071. Epub 2016 Dec 22 [PubMed PMID: 28012747]
Barry MJ, Fowler FJ Jr, O'Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, Cockett AT. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. The Journal of urology. 1992 Nov:148(5):1549-57; discussion 1564 [PubMed PMID: 1279218]
Dougherty JM, Leslie SW, Aeddula NR. Male Urinary Retention: Acute and Chronic. StatPearls. 2025 Jan:(): [PubMed PMID: 30860734]
Bohnen AM, Groeneveld FP, Bosch JL. Serum prostate-specific antigen as a predictor of prostate volume in the community: the Krimpen study. European urology. 2007 Jun:51(6):1645-52; discussion 1652-3 [PubMed PMID: 17320271]
Shim HB, Kim YD, Jung TY, Lee JK, Ku JH. Prostate-specific antigen and prostate volume in Korean men with spinal cord injury: a case-control study. Spinal cord. 2008 Jan:46(1):11-5 [PubMed PMID: 17387315]
Level 2 (mid-level) evidencePark DS, Oh JJ, Hong JY, Hong YK, Choi DK, Gong IH, Hwang JH, Kwon SW. Serum prostate-specific antigen as a predictor of prostate volume and lower urinary tract symptoms in a community-based cohort: a large-scale Korean screening study. Asian journal of andrology. 2013 Mar:15(2):249-53. doi: 10.1038/aja.2012.132. Epub 2013 Jan 28 [PubMed PMID: 23353717]
Level 2 (mid-level) evidencePark DS, Hong JY, Hong YK, Lee SR, Hwang JH, Kang MH, Kwon SW, Oh JJ. Correlation between serum prostate specific antigen level and prostate volume in a community-based cohort: large-scale screening of 35,223 Korean men. Urology. 2013 Dec:82(6):1394-9. doi: 10.1016/j.urology.2013.07.071. Epub [PubMed PMID: 24295251]
Level 2 (mid-level) evidenceRoehrborn CG, Boyle P, Gould AL, Waldstreicher J. Serum prostate-specific antigen as a predictor of prostate volume in men with benign prostatic hyperplasia. Urology. 1999 Mar:53(3):581-9 [PubMed PMID: 10096388]
David MK, Leslie SW. Prostate-Specific Antigen. StatPearls. 2024 Jan:(): [PubMed PMID: 32491427]
Abrams P. Objective evaluation of bladder outlet obstruction. British journal of urology. 1995 Jul:76 Suppl 1():11-5 [PubMed PMID: 7544210]
Nitti VW. Pressure flow urodynamic studies: the gold standard for diagnosing bladder outlet obstruction. Reviews in urology. 2005:7 Suppl 6(Suppl 6):S14-21 [PubMed PMID: 16986024]
Leslie SW, Sajjad H, Sharma S. Postobstructive Diuresis. StatPearls. 2024 Jan:(): [PubMed PMID: 29083564]
Speakman MJ, Cheng X. Management of the complications of BPH/BOO. Indian journal of urology : IJU : journal of the Urological Society of India. 2014 Apr:30(2):208-13. doi: 10.4103/0970-1591.127856. Epub [PubMed PMID: 24744522]
Roehrborn CG, Bruskewitz R, Nickel GC, Glickman S, Cox C, Anderson R, Kandzari S, Herlihy R, Kornitzer G, Brown BT, Holtgrewe HL, Taylor A, Wang D, Waldstreicher J. Urinary retention in patients with BPH treated with finasteride or placebo over 4 years. Characterization of patients and ultimate outcomes. The PLESS Study Group. European urology. 2000 May:37(5):528-36 [PubMed PMID: 10765090]
Level 1 (high-level) evidenceBoettcher S, Brandt AS, Roth S, Mathers MJ, Lazica DA. Urinary retention: benefit of gradual bladder decompression - myth or truth? A randomized controlled trial. Urologia internationalis. 2013:91(2):140-4. doi: 10.1159/000350943. Epub 2013 Jul 16 [PubMed PMID: 23859894]
Level 1 (high-level) evidenceHelman TA, Browne BM. Advances in Outpatient Therapies and Treatment of Benign Prostatic Hyperplasia: A Comprehensive Review for Men's Health. The Medical clinics of North America. 2024 Sep:108(5):981-991. doi: 10.1016/j.mcna.2024.03.009. Epub 2024 May 3 [PubMed PMID: 39084845]
Level 3 (low-level) evidenceDjavan B, Fong YK, Harik M, Milani S, Reissigl A, Chaudry A, Anagnostou T, Bagheri F, Waldert M, Kreuzer S, Fajkovic H, Marberger M. Longitudinal study of men with mild symptoms of bladder outlet obstruction treated with watchful waiting for four years. Urology. 2004 Dec:64(6):1144-8 [PubMed PMID: 15596187]
Emberton M, Cornel EB, Bassi PF, Fourcade RO, Gómez JM, Castro R. Benign prostatic hyperplasia as a progressive disease: a guide to the risk factors and options for medical management. International journal of clinical practice. 2008 Jul:62(7):1076-86. doi: 10.1111/j.1742-1241.2008.01785.x. Epub 2008 May 8 [PubMed PMID: 18479366]
Nachawati D, Patel JB. Alpha-Blockers. StatPearls. 2024 Jan:(): [PubMed PMID: 32310526]
Hirahara N, Harikai S, Fujihara A, Yamada Y, Ushijima S, Ukimura O, KPUM-LUTS Research Group. Efficacy of tadalafil on symptom-specific bother in men with lower urinary tract symptoms. Lower urinary tract symptoms. 2022 Sep:14(5):393-400. doi: 10.1111/luts.12457. Epub 2022 Jul 13 [PubMed PMID: 35830962]
Kuno T, Tamura K, Fukuhara H, Fukata S, Ashida S, Karashima T, Sawada K, Yasuda M, Watanabe H, Komatsu F, Kuroiwa H, Saito M, Inoue K. Tadalafil 5 mg Once Daily Improved Each IPSS Subscore, QOL, and Nocturia in Elderly BPH Patients over 70 Years Old in a Real-World Clinical Setting. Urologia internationalis. 2022:106(10):1005-1011. doi: 10.1159/000519476. Epub 2021 Oct 21 [PubMed PMID: 34673648]
Cui J, Cao D, Bai Y, Wang J, Yin S, Wei W, Xiao Y, Wang J, Wei Q. Efficacy and Safety of 12-week Monotherapy With Once Daily 5 mg Tadalafil for Lower Urinary Tract Symptoms of Benign Prostatic Hyperplasia: Evidence-based Analysis. Frontiers in medicine. 2021:8():744012. doi: 10.3389/fmed.2021.744012. Epub 2021 Oct 12 [PubMed PMID: 34712682]
Dekalo S, McArthur E, Campbell J, Ordon M, Power N, Welk B. 5α-reductase inhibitors and the risk of bladder cancer in a large, population-based cohort. Urologic oncology. 2023 Jan:41(1):50.e11-50.e17. doi: 10.1016/j.urolonc.2022.09.004. Epub 2022 Oct 29 [PubMed PMID: 36319553]
Ahmad MS, Dar YA, Khawaja AR, Para SA, Malik SA, Wani MS, Bhat AH, Wani PM. Comparative study of tamsulosin versus tadalafil in benign prostatic hyperplasia patients with lower urinary tract symptoms. A prospective randomized study. Urology annals. 2022 Jul-Sep:14(3):236-240. doi: 10.4103/ua.ua_6_21. Epub 2022 Apr 14 [PubMed PMID: 36117785]
Level 1 (high-level) evidenceGuo B, Chen X, Wang M, Hou H, Zhang Z, Liu M. Comparative Effectiveness of Tadalafil versus Tamsulosin in Treating Lower Urinary Tract Symptoms Suggestive of Benign Prostate Hyperplasia: A Meta-Analysis of Randomized Controlled Trials. Medical science monitor : international medical journal of experimental and clinical research. 2020 Apr 24:26():e923179. doi: 10.12659/MSM.923179. Epub 2020 Apr 24 [PubMed PMID: 32327621]
Level 1 (high-level) evidenceSebastianelli A, Spatafora P, Morselli S, Vignozzi L, Serni S, McVary KT, Kaplan S, Gravas S, Chapple C, Gacci M. Tadalafil Alone or in Combination with Tamsulosin for the Management for LUTS/BPH and ED. Current urology reports. 2020 Oct 27:21(12):56. doi: 10.1007/s11934-020-01009-7. Epub 2020 Oct 27 [PubMed PMID: 33108544]
Sun K, Sun F, Yao H, Zhang D, Wu G, Wang T, Wang J, Wu J. Efficacy and Safety of Combination Comprising Tamsulosin and PDE5-Is, Relative to Monotherapies, in Treating Lower Urinary Tract Symptoms and Erectile Dysfunction Associated With Benign Prostatic Hyperplasia: A Meta-Analysis. American journal of men's health. 2020 Nov-Dec:14(6):1557988320980180. doi: 10.1177/1557988320980180. Epub [PubMed PMID: 33342335]
Level 1 (high-level) evidenceSeidensticker M, Tasch S, Mietens A, Exintaris B, Middendorff R. Treatment of benign prostatic hyperplasia and abnormal ejaculation: live imaging reveals tamsulosin - but not tadalafil - induced dysfunction of prostate, seminal vesicles and epididymis. Reproduction (Cambridge, England). 2022 Dec 1:164(6):291-301. doi: 10.1530/REP-22-0197. Epub 2022 Nov 7 [PubMed PMID: 36173812]
Honda M, Kimura Y, Teraoka S, Kawamoto B, Morizane S, Hikita K, Takenaka A. Efficacy of Combination Treatment with Tadalafil and Mirabegron in Patients with Benign Prostatic Hyperplasia Who Presented with Persistent Storage Symptoms After Tadalafil Monotreatment: A Prospective, Multicenter, Open-Labeled Study. Yonago acta medica. 2022 Aug:65(3):231-237. doi: 10.33160/yam.2022.08.009. Epub 2022 Aug 4 [PubMed PMID: 36061573]
Fahmy G, Hess J. Tadalafil. StatPearls. 2024 Jan:(): [PubMed PMID: 38753929]
Gormley GJ, Stoner E, Bruskewitz RC, Imperato-McGinley J, Walsh PC, McConnell JD, Andriole GL, Geller J, Bracken BR, Tenover JS. The effect of finasteride in men with benign prostatic hyperplasia. The Finasteride Study Group. The New England journal of medicine. 1992 Oct 22:327(17):1185-91 [PubMed PMID: 1383816]
Level 1 (high-level) evidenceRittmaster RS, Norman RW, Thomas LN, Rowden G. Evidence for atrophy and apoptosis in the prostates of men given finasteride. The Journal of clinical endocrinology and metabolism. 1996 Feb:81(2):814-9 [PubMed PMID: 8636309]
Level 1 (high-level) evidenceLi Y, Ma J, Qin XH, Hu CY. The efficacy and safety of dutasteride and finasteride in patients with benign prostatic hyperplasia: a systematic review and meta-analysis. Translational andrology and urology. 2022 Mar:11(3):313-324. doi: 10.21037/tau-22-58. Epub [PubMed PMID: 35402192]
Level 1 (high-level) evidenceBansal A, Arora A. Transurethral Resection of Prostate and Bleeding: A Prospective, Randomized, Double-Blind Placebo-Controlled Trial to See the Efficacy of Short-Term Use of Finasteride and Dutasteride on Operative Blood Loss and Prostatic Microvessel Density. Journal of endourology. 2017 Sep:31(9):910-917. doi: 10.1089/end.2016.0696-rev. Epub 2017 Jun 26 [PubMed PMID: 28650680]
Level 1 (high-level) evidenceRoehrborn CG, Siami P, Barkin J, Damião R, Major-Walker K, Nandy I, Morrill BB, Gagnier RP, Montorsi F, CombAT Study Group. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study. European urology. 2010 Jan:57(1):123-31. doi: 10.1016/j.eururo.2009.09.035. Epub 2009 Sep 19 [PubMed PMID: 19825505]
Level 1 (high-level) evidenceRegadas RP, Reges R, Cerqueira JB, Sucupira DG, Josino IR, Nogueira EA, Jamacaru FV, de Moraes MO, Silva LF. Urodynamic effects of the combination of tamsulosin and daily tadalafil in men with lower urinary tract symptoms secondary to benign prostatic hyperplasia: a randomized, placebo-controlled clinical trial. International urology and nephrology. 2013 Feb:45(1):39-43. doi: 10.1007/s11255-012-0317-7. Epub 2012 Oct 30 [PubMed PMID: 23108604]
Level 1 (high-level) evidenceGarg H, Wheeler KM, Dursun F, Cooper RE, Pruthi DK, Kaushik D, Thompson IM, Svatek RS, Liss MA. Impact of Finasteride on Survival in Bladder Cancer: A Retrospective Multi-institutional Database Analysis. Clinical genitourinary cancer. 2023 Apr:21(2):314.e1-314.e7. doi: 10.1016/j.clgc.2022.10.014. Epub 2022 Oct 29 [PubMed PMID: 36402643]
Level 2 (mid-level) evidenceXiang P, Du Z, Hao Y, Guan D, Liu D, Yan W, Wang M, Liu Y, Ping H. Impact of Androgen Suppression Therapy on the Risk and Prognosis of Bladder Cancer: A Systematic Review and Meta-Analysis. Frontiers in oncology. 2021:11():784627. doi: 10.3389/fonc.2021.784627. Epub 2021 Dec 14 [PubMed PMID: 34970495]
Level 1 (high-level) evidenceZhu D, Srivastava A, Agalliu I, Fram E, Kovac EZ, Aboumohamed A, Schoenberg MP, Sankin AI. Finasteride Use and Risk of Bladder Cancer in a Multiethnic Population. The Journal of urology. 2021 Jul:206(1):15-21. doi: 10.1097/JU.0000000000001694. Epub 2021 Feb 22 [PubMed PMID: 33617325]
Björnebo L, Nordström T, Discacciati A, Palsdottir T, Aly M, Grönberg H, Eklund M, Lantz A. Association of 5α-Reductase Inhibitors With Prostate Cancer Mortality. JAMA oncology. 2022 Jul 1:8(7):1019-1026. doi: 10.1001/jamaoncol.2022.1501. Epub [PubMed PMID: 35587340]
Zito PM, Bistas KG, Patel P, Syed K. Finasteride. StatPearls. 2024 Jan:(): [PubMed PMID: 30020701]
Ghossein N, Kang M, Lakhkar AD. Anticholinergic Medications. StatPearls. 2024 Jan:(): [PubMed PMID: 32310353]
de la Rosette JJ, Alivizatos G, Madersbacher S, Perachino M, Thomas D, Desgrandchamps F, de Wildt M, European Association of Urology. EAU Guidelines on benign prostatic hyperplasia (BPH). European urology. 2001 Sep:40(3):256-63; discussion 264 [PubMed PMID: 11684840]
Leslie SW, Chargui S, Stormont G. Transurethral Resection of the Prostate. StatPearls. 2025 Jan:(): [PubMed PMID: 32809719]
Lerner LB, McVary KT, Barry MJ, Bixler BR, Dahm P, Das AK, Gandhi MC, Kaplan SA, Kohler TS, Martin L, Parsons JK, Roehrborn CG, Stoffel JT, Welliver C, Wilt TJ. Management of Lower Urinary Tract Symptoms Attributed to Benign Prostatic Hyperplasia: AUA GUIDELINE PART II-Surgical Evaluation and Treatment. The Journal of urology. 2021 Oct:206(4):818-826. doi: 10.1097/JU.0000000000002184. Epub 2021 Aug 13 [PubMed PMID: 34384236]
Orandi A. Transurethral incision of prostate compared with transurethral resection of prostate in 132 matching cases. The Journal of urology. 1987 Oct:138(4):810-5 [PubMed PMID: 2443728]
Level 3 (low-level) evidenceWroclawski ML, Carneiro A, Amarante RD, Oliveira CE, Shimanoe V, Bianco BA, Sakuramoto PK, Pompeo AC. 'Button type' bipolar plasma vaporisation of the prostate compared with standard transurethral resection: a systematic review and meta-analysis of short-term outcome studies. BJU international. 2016 Apr:117(4):662-8. doi: 10.1111/bju.13255. Epub 2015 Oct 3 [PubMed PMID: 26299915]
Level 1 (high-level) evidenceGeavlete B, Multescu R, Dragutescu M, Jecu M, Georgescu D, Geavlete P. Transurethral resection (TUR) in saline plasma vaporization of the prostate vs standard TUR of the prostate: 'the better choice' in benign prostatic hyperplasia? BJU international. 2010 Dec:106(11):1695-9. doi: 10.1111/j.1464-410X.2010.09433.x. Epub [PubMed PMID: 20518763]
Level 2 (mid-level) evidenceXiao K, Ma Y, Luo Z, Li H, Jin T. Network meta-analysis of the treatment safety and efficacy of different energy systems in prostate vaporization. Lasers in medical science. 2023 Jun 28:38(1):150. doi: 10.1007/s10103-023-03781-7. Epub 2023 Jun 28 [PubMed PMID: 37378687]
Level 1 (high-level) evidenceBenejam Gual J, Servera Ruiz de Velasco A, Hernández Martínez Y, García-Miralles Grávalos R. [Greenlight laser vaporization: Past, present and future?]. Archivos espanoles de urologia. 2020 Oct:73(8):675-681 [PubMed PMID: 33025912]
Kuntz RM, Lehrich K, Ahyai SA. Holmium laser enucleation of the prostate versus open prostatectomy for prostates greater than 100 grams: 5-year follow-up results of a randomised clinical trial. European urology. 2008 Jan:53(1):160-6 [PubMed PMID: 17869409]
Level 1 (high-level) evidenceMostafa MM, Patil N, Khalil M, Elgammal MA, Mahdy A. Is Holmium Laser Enucleation of Prostate equally effective in management of benign prostatic hyperplasia patients with either voiding or storage lower urinary tract symptoms? A comparative study. Archivio italiano di urologia, andrologia : organo ufficiale [di] Societa italiana di ecografia urologica e nefrologica. 2022 Jun 29:94(2):174-179. doi: 10.4081/aiua.2022.2.174. Epub 2022 Jun 29 [PubMed PMID: 35775342]
Level 2 (mid-level) evidenceYin L, Teng J, Huang CJ, Zhang X, Xu D. Holmium laser enucleation of the prostate versus transurethral resection of the prostate: a systematic review and meta-analysis of randomized controlled trials. Journal of endourology. 2013 May:27(5):604-11. doi: 10.1089/end.2012.0505. Epub 2013 Feb 1 [PubMed PMID: 23167266]
Level 1 (high-level) evidenceGilling P, Anderson P, Tan A. Aquablation of the Prostate for Symptomatic Benign Prostatic Hyperplasia: 1-Year Results. The Journal of urology. 2017 Jun:197(6):1565-1572. doi: 10.1016/j.juro.2017.01.056. Epub 2017 Jan 20 [PubMed PMID: 28111300]
Taktak S, Jones P, Haq A, Rai BP, Somani BK. Aquablation: a novel and minimally invasive surgery for benign prostate enlargement. Therapeutic advances in urology. 2018 Jun:10(6):183-188. doi: 10.1177/1756287218760518. Epub 2018 Feb 26 [PubMed PMID: 29899759]
Level 3 (low-level) evidenceZorn KC, Chakraborty A, Chughtai B, Mehan R, Elterman D, Nguyen DD, Bouhadana D, Glaser AP, Marhamati S, Barber N, Helfand BT. Safety and Efficacy of Same Day Discharge for Men Undergoing Contemporary Robotic-assisted Aquablation Prostate Surgery in an Ambulatory Surgery Center Setting-First Global Experience. Urology. 2024 Aug 17:():. pii: S0090-4295(24)00658-7. doi: 10.1016/j.urology.2024.08.006. Epub 2024 Aug 17 [PubMed PMID: 39159759]
Nguyen DD, Barber N, Bidair M, Gilling P, Anderson P, Zorn KC, Badlani G, Humphreys M, Kaplan S, Kaufman R, So A, Paterson R, Goldenberg L, Elterman D, Desai M, Lingeman J, Roehrborn C, Bhojani N. Waterjet Ablation Therapy for Endoscopic Resection of prostate tissue trial (WATER) vs WATER II: comparing Aquablation therapy for benign prostatic hyperplasia in 30-80 and 80-150 mL prostates. BJU international. 2020 Jan:125(1):112-122. doi: 10.1111/bju.14917. Epub 2019 Nov 8 [PubMed PMID: 31599044]
Helfand BT, Kasraeian A, Sterious S, Glaser AP, Talaty P, Alcantara M, Alcantara KM, Higgins A, Ghiraldi E, Elterman DS. How I do it: Aquablation in very large prostates (} 150 mL). The Canadian journal of urology. 2022 Apr:29(2):11111-11115 [PubMed PMID: 35429430]
Helfand BT, Glaser AP, Kasraeian A, Sterious S, Talaty P, Alcantara M, Alcantara KM, Higgins A, Ghiraldi E, Elterman D. Men with lower urinary tract symptoms secondary to BPH undergoing Aquablation with very large prostates (} 150 mL). The Canadian journal of urology. 2021 Dec:28(6):10884-10888 [PubMed PMID: 34895392]
Gilling PJ, Barber N, Bidair M, Anderson P, Sutton M, Aho T, Kramolowsky E, Thomas A, Kaufman RP Jr, Badlani G, Plante M, Desai M, Doumanian L, Te AE, Roehrborn CG. Five-year outcomes for Aquablation therapy compared to TURP: results from a double-blind, randomized trial in men with LUTS due to BPH. The Canadian journal of urology. 2022 Feb:29(1):10960-10968 [PubMed PMID: 35150215]
Level 1 (high-level) evidenceFiori C, Checcucci E, Gilling P, Amparore D, Volpi G, De Cillis S, Aimar R, Sica M, Cattaneo G, Alleva G, Manfredi M, Porpiglia F, ESUT Lower Tract Group. All you need to know about "Aquablation" procedure for treatment of benign prostatic obstruction. Minerva urologica e nefrologica = The Italian journal of urology and nephrology. 2020 Apr:72(2):152-161. doi: 10.23736/S0393-2249.20.03654-1. Epub 2020 Feb 19 [PubMed PMID: 32083415]
Elterman D, Gilling P, Roehrborn C, Barber N, Misrai V, Zorn KC, Bhojani N, Te A, Humphreys M, Kaplan S, Desai M, Bach T. Meta-analysis with individual data of functional outcomes following Aquablation for lower urinary tract symptoms due to BPH in various prostate anatomies. BMJ surgery, interventions, & health technologies. 2021:3(1):e000090. doi: 10.1136/bmjsit-2021-000090. Epub 2021 Jun 23 [PubMed PMID: 35047807]
Level 1 (high-level) evidenceOumedjbeur K, Corsi NJ, Bouhadana D, Ibrahim A, Nguyen DD, Matta I, Arezki A, Sadri I, Elsherbini T, Bhojani N, Elterman DS, Chughtai B, Helfand BT, Glaser AP, Misrai V, Kaplan S, Gilling P, Barber N, Desai M, Badlani GH, Te AE, Roehrborn CG, Zorn KC. Aquablation versus TURP: 5-year outcomes of the WATER randomized clinical trial for prostate volumes 50-80 mL. The Canadian journal of urology. 2023 Oct:30(5):11650-11658 [PubMed PMID: 37838991]
Level 1 (high-level) evidencePlante M, Gilling P, Barber N, Bidair M, Anderson P, Sutton M, Aho T, Kramolowsky E, Thomas A, Cowan B, Kaufman RP Jr, Trainer A, Arther A, Badlani G, Desai M, Doumanian L, Te AE, DeGuenther M, Roehrborn C. Symptom relief and anejaculation after aquablation or transurethral resection of the prostate: subgroup analysis from a blinded randomized trial. BJU international. 2019 Apr:123(4):651-660. doi: 10.1111/bju.14426. Epub 2018 Jul 26 [PubMed PMID: 29862630]
Level 1 (high-level) evidenceBurton CS, Dobberfuhl AD, Comiter CV. Outcomes of Aquablation in Men With Acute and Chronic Urinary Retention. Urology. 2023 Oct:180():214-218. doi: 10.1016/j.urology.2023.06.028. Epub 2023 Jul 11 [PubMed PMID: 37442297]
Yee CH, Tang SF, Yuen SK, Chan CK, Teoh JYC, Chiu PKF, Ng CF. Technique, outcome and changes in prostate dimensions in patients with urinary retention managed by aquablation. International urology and nephrology. 2022 Aug:54(8):1787-1792. doi: 10.1007/s11255-022-03244-y. Epub 2022 May 27 [PubMed PMID: 35622268]
Gilling P, Barber N, Bidair M, Anderson P, Sutton M, Aho T, Kramolowsky E, Thomas A, Cowan B, Kaufman RP Jr, Trainer A, Arther A, Badlani G, Plante M, Desai M, Doumanian L, Te AE, DeGuenther M, Roehrborn C. WATER: A Double-Blind, Randomized, Controlled Trial of Aquablation(®) vs Transurethral Resection of the Prostate in Benign Prostatic Hyperplasia. The Journal of urology. 2018 May:199(5):1252-1261. doi: 10.1016/j.juro.2017.12.065. Epub 2018 Jan 31 [PubMed PMID: 29360529]
Level 1 (high-level) evidenceGilling P, Reuther R, Kahokehr A, Fraundorfer M. Aquablation - image-guided robot-assisted waterjet ablation of the prostate: initial clinical experience. BJU international. 2016 Jun:117(6):923-9. doi: 10.1111/bju.13358. Epub 2015 Nov 19 [PubMed PMID: 26477826]
Misrai V, Rijo E, Zorn KC, Barry-Delongchamps N, Descazeaud A. Waterjet Ablation Therapy for Treating Benign Prostatic Obstruction in Patients with Small- to Medium-size Glands: 12-month Results of the First French Aquablation Clinical Registry. European urology. 2019 Nov:76(5):667-675. doi: 10.1016/j.eururo.2019.06.024. Epub 2019 Jul 5 [PubMed PMID: 31281024]
Whiting D, Ng KL, Barber N. Initial single centre experience of Aquablation of the prostate using the AquaBeam system with athermal haemostasis for the treatment of benign prostatic hyperplasia: 1-year outcomes. World journal of urology. 2021 Aug:39(8):3019-3024. doi: 10.1007/s00345-020-03534-z. Epub 2021 Jan 3 [PubMed PMID: 33392647]
Hwang EC, Jung JH, Borofsky M, Kim MH, Dahm P. Aquablation of the prostate for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia. The Cochrane database of systematic reviews. 2019 Feb 13:2(2):CD013143. doi: 10.1002/14651858.CD013143.pub2. Epub 2019 Feb 13 [PubMed PMID: 30759311]
Level 1 (high-level) evidenceAwosika AO, Farrar MC, Jacobs TF. Paclitaxel. StatPearls. 2024 Jan:(): [PubMed PMID: 30725602]
Weaver BA. How Taxol/paclitaxel kills cancer cells. Molecular biology of the cell. 2014 Sep 15:25(18):2677-81. doi: 10.1091/mbc.E14-04-0916. Epub [PubMed PMID: 25213191]
Level 3 (low-level) evidenceHerten M, Torsello GB, Schönefeld E, Stahlhoff S. Critical appraisal of paclitaxel balloon angioplasty for femoral-popliteal arterial disease. Vascular health and risk management. 2016:12():341-56. doi: 10.2147/VHRM.S81122. Epub 2016 Aug 29 [PubMed PMID: 27621646]
Chhabra L, Zain MA, Siddiqui WJ. Coronary Stents. StatPearls. 2024 Jan:(): [PubMed PMID: 29939581]
Chhabra L, Zain MA, Siddiqui WJ. Angioplasty. StatPearls. 2024 Jan:(): [PubMed PMID: 29763069]
Majeed H, Chowdhury YS. Percutaneous Transluminal Angioplasty and Balloon Catheters. StatPearls. 2024 Jan:(): [PubMed PMID: 33351412]
Valdes PJ, Akbar H, Kahloon RA, Diaz MA. Intracoronary Stents. StatPearls. 2024 Jan:(): [PubMed PMID: 29939565]
Kaplan SA, Pichardo M, Rijo E, Espino G, Lay RR, Estrella R. One-year outcomes after treatment with a drug-coated balloon catheter system for lower urinary tract symptoms related to benign prostatic hyperplasia. Prostate cancer and prostatic diseases. 2021 Dec:24(4):1073-1079. doi: 10.1038/s41391-021-00362-z. Epub 2021 Apr 8 [PubMed PMID: 33833379]
Elterman DS, Gao B, Zorn KC, Bhojani N, Te A, Chughtai B, Kaplan SA. How I Do It: Optilume BPH catheter system. The Canadian journal of urology. 2023 Jun:30(3):11568-11573 [PubMed PMID: 37344470]
Donatucci CF, Berger N, Kreder KJ, Donohue RE, Raife MJ, Crawford ED. Randomized clinical trial comparing balloon dilatation to transurethral resection of prostate for benign prostatic hyperplasia. Urology. 1993 Jul:42(1):42-9 [PubMed PMID: 7687079]
Level 1 (high-level) evidenceRenfer L, Thompson IM, Desmond PM, Zeidman EJ, Mueller EJ. Balloon dilation of the prostate: correlation with magnetic resonance imaging and transrectal ultrasound findings. Journal of endourology. 1995 Jun:9(3):283-6 [PubMed PMID: 7550276]
Saporta L, Aridogan IA, Erlich N, Yachia D. Objective and subjective comparison of transurethral resection, transurethral incision and balloon dilatation of the prostate. A prospective study. European urology. 1996:29(4):439-45 [PubMed PMID: 8791051]
Level 1 (high-level) evidenceChiou RK, Binard JE, Ebersole ME, Horan JJ, Chiou YK, Lynch B. Randomized comparison of balloon dilation and transurethral incision for treatment of symptomatic benign prostatic hyperplasia. Journal of endourology. 1994 Jun:8(3):221-4 [PubMed PMID: 7524917]
Level 1 (high-level) evidenceGarcia C, Chin P, Rashid P, Woo HH. Prostatic urethral lift: A minimally invasive treatment for benign prostatic hyperplasia. Prostate international. 2015 Mar:3(1):1-5. doi: 10.1016/j.prnil.2015.02.002. Epub 2015 Feb 13 [PubMed PMID: 26157759]
Rahman A, Leslie SW, Desai D. Prostatic Urethral Lift. StatPearls. 2024 Jan:(): [PubMed PMID: 39163438]
Martinelli E, Cindolo L, Grossi FS, Kuczyk MA, Siena G, Oelke M. Transurethral water vapor ablation of the prostate with the Rezūm system: Urodynamic findings. Neurourology and urodynamics. 2023 Jan:42(1):249-255. doi: 10.1002/nau.25076. Epub 2022 Nov 6 [PubMed PMID: 36335610]
McVary KT, Gange SN, Gittelman MC, Goldberg KA, Patel K, Shore ND, Levin RM, Rousseau M, Beahrs JR, Kaminetsky J, Cowan BE, Cantrill CH, Mynderse LA, Ulchaker JC, Larson TR, Dixon CM, Roehrborn CG. Minimally Invasive Prostate Convective Water Vapor Energy Ablation: A Multicenter, Randomized, Controlled Study for the Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia. The Journal of urology. 2016 May:195(5):1529-1538. doi: 10.1016/j.juro.2015.10.181. Epub 2015 Nov 22 [PubMed PMID: 26614889]
Level 1 (high-level) evidenceMcVary KT, Gittelman MC, Goldberg KA, Patel K, Shore ND, Levin RM, Pliskin M, Beahrs JR, Prall D, Kaminetsky J, Cowan BE, Cantrill CH, Mynderse LA, Ulchaker JC, Tadros NN, Gange SN, Roehrborn CG. Final 5-Year Outcomes of the Multicenter Randomized Sham-Controlled Trial of a Water Vapor Thermal Therapy for Treatment of Moderate to Severe Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia. The Journal of urology. 2021 Sep:206(3):715-724. doi: 10.1097/JU.0000000000001778. Epub 2021 Apr 19 [PubMed PMID: 33872051]
Level 1 (high-level) evidenceMynderse LA, Hanson D, Robb RA, Pacik D, Vit V, Varga G, Wagrell L, Tornblom M, Cedano ER, Woodrum DA, Dixon CM, Larson TR. Rezūm System Water Vapor Treatment for Lower Urinary Tract Symptoms/Benign Prostatic Hyperplasia: Validation of Convective Thermal Energy Transfer and Characterization With Magnetic Resonance Imaging and 3-Dimensional Renderings. Urology. 2015 Jul:86(1):122-7. doi: 10.1016/j.urology.2015.03.021. Epub 2015 May 16 [PubMed PMID: 25987496]
Level 1 (high-level) evidenceTadrist A, Baboudjian M, Bah MB, Alegorides C, Bottet F, Arroua F, Eghazarian C, Fourmarier M. Water vapor thermal therapy for indwelling urinary catheter removal in frail patients. International urology and nephrology. 2023 Feb:55(2):249-253. doi: 10.1007/s11255-022-03408-w. Epub 2022 Nov 7 [PubMed PMID: 36342555]
Cindolo L, Campobasso D, Conti E, Uricchio F, Franzoso F, Maruzzi D, Viola L, Varvello F, Balsamo R, Ferrari G, Morselli S, Siena G. Do Patients Treated with Water Vapor Therapy and Meeting Randomized Clinical Trial Criteria Have Better Urinary and Sexual Outcomes Than an Unselected Cohort? Journal of endourology. 2023 Mar:37(3):323-329. doi: 10.1089/end.2022.0637. Epub 2022 Dec 20 [PubMed PMID: 36453237]
Level 1 (high-level) evidenceUlchaker JC, Martinson MS. Cost-effectiveness analysis of six therapies for the treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. ClinicoEconomics and outcomes research : CEOR. 2018:10():29-43. doi: 10.2147/CEOR.S148195. Epub 2017 Dec 29 [PubMed PMID: 29343977]
Elterman D, Bhojani N, Vannabouathong C, Chughtai B, Zorn KC. Rezūm therapy for ≥80-mL benign prostatic enlargement: a large, multicentre cohort study. BJU international. 2022 Oct:130(4):522-527. doi: 10.1111/bju.15753. Epub 2022 May 7 [PubMed PMID: 35466513]
Munien K, Leslie SW, Desai D. Water Vapor Thermal Ablation of the Prostate. StatPearls. 2025 Jan:(): [PubMed PMID: 38261694]
Franco JVA, Garegnani L, Escobar Liquitay CM, Borofsky M, Dahm P. Transurethral Microwave Thermotherapy for Benign Prostatic Hyperplasia: An Updated Cochrane Review. The world journal of men's health. 2022 Jan:40(1):127-138. doi: 10.5534/wjmh.210115. Epub 2021 Aug 4 [PubMed PMID: 34448377]
Yu H, Isaacson AJ, Burke CT. Review of Current Literature for Prostatic Artery Embolization. Seminars in interventional radiology. 2016 Sep:33(3):231-5. doi: 10.1055/s-0036-1586141. Epub [PubMed PMID: 27582611]
Mirakhur A, McWilliams JP. Prostate Artery Embolization for Benign Prostatic Hyperplasia: Current Status. Canadian Association of Radiologists journal = Journal l'Association canadienne des radiologistes. 2017 Feb:68(1):84-89. doi: 10.1016/j.carj.2016.06.003. Epub 2016 Nov 22 [PubMed PMID: 27887933]
Petrillo M, Pesapane F, Fumarola EM, Emili I, Acquasanta M, Patella F, Angileri SA, Rossi UG, Piacentini I, Granata AM, Ierardi AM, Carrafiello G. State of the art of prostatic arterial embolization for benign prostatic hyperplasia. Gland surgery. 2018 Apr:7(2):188-199. doi: 10.21037/gs.2018.03.01. Epub [PubMed PMID: 29770312]
Veyg D, Mohanka R, Rumball IP, Liang R, Garcia-Reyes K, Bishay V, Fischman AM. Comparison of 24-Month Clinical Outcomes after Prostatic Artery Embolization in Prostate Glands Larger versus Smaller than 80 mL: A Systematic Review. Journal of vascular and interventional radiology : JVIR. 2023 Apr:34(4):578-584.e1. doi: 10.1016/j.jvir.2022.11.025. Epub 2022 Dec 5 [PubMed PMID: 36470516]
Level 1 (high-level) evidenceRaizenne BL, Zheng X, Oumedjbeur K, Mao J, Zorn KC, Elterman D, Bhojani N, McClure T, Te A, Kaplan S, Sedrakyan A, Chughtai B. Prostatic artery embolization compared to transurethral resection of the prostate and prostatic urethral lift: a real-world population-based study. World journal of urology. 2023 Jan:41(1):179-188. doi: 10.1007/s00345-022-04218-6. Epub 2022 Dec 4 [PubMed PMID: 36463348]
Zhou G, He J, Huang G, Ren L, Zhuge W, Wang W. Efficacy and Safety of Transurethral Columnar Balloon Dilation of the Prostate for the Treatment of Benign Prostatic Hyperplasia: A Multicenter Trial. Computational and mathematical methods in medicine. 2022:2022():7881247. doi: 10.1155/2022/7881247. Epub 2022 Jun 9 [PubMed PMID: 35720037]
Level 1 (high-level) evidenceHuang W, Guo Y, Xiao G, Qin X. Treatment of benign prostatic hyperplasia using transurethral split of the prostate with a columnar balloon catheter. Journal of endourology. 2015 Mar:29(3):344-50. doi: 10.1089/end.2014.0207. Epub 2014 Dec 4 [PubMed PMID: 25285775]
Level 3 (low-level) evidenceZhang DP, Pan ZB, Zhang HT. Urinary and sexual function changes in benign prostatic hyperplasia patients before and after transurethral columnar balloon dilatation of the prostate. World journal of clinical cases. 2022 Jul 16:10(20):6794-6802. doi: 10.12998/wjcc.v10.i20.6794. Epub [PubMed PMID: 36051138]
Level 3 (low-level) evidenceJacobsen SJ, Jacobson DJ, Girman CJ, Roberts RO, Rhodes T, Guess HA, Lieber MM. Natural history of prostatism: risk factors for acute urinary retention. The Journal of urology. 1997 Aug:158(2):481-7 [PubMed PMID: 9224329]
Level 2 (mid-level) evidenceNickel JC. Benign prostatic hyperplasia: does prostate size matter? Reviews in urology. 2003:5 Suppl 4(Suppl 4):S12-7 [PubMed PMID: 16985958]
Pickard R, Emberton M, Neal DE. The management of men with acute urinary retention. National Prostatectomy Audit Steering Group. British journal of urology. 1998 May:81(5):712-20 [PubMed PMID: 9634047]
Level 2 (mid-level) evidenceKashif KM, Foley SJ, Basketter V, Holmes SA. Haematuria associated with BPH-Natural history and a new treatment option. Prostate cancer and prostatic diseases. 1998 Mar:1(3):154-156 [PubMed PMID: 12496909]
Breyer BN, Kim SK, Kirkby E, Marianes A, Vanni AJ, Westney OL. Updates to Incontinence After Prostate Treatment: AUA/GURS/SUFU Guideline (2024). The Journal of urology. 2024 Oct:212(4):531-538. doi: 10.1097/JU.0000000000004088. Epub 2024 Jun 27 [PubMed PMID: 38934789]
Klock JA, Palacios AR, Leslie SW, Feloney MP. Artificial Urinary Sphincters and Adjustable Dual-Balloon Continence Therapy in Men. StatPearls. 2024 Jan:(): [PubMed PMID: 37983344]
Thomas S. Good practice in continence services. Nursing standard (Royal College of Nursing (Great Britain) : 1987). 2000 Aug 9-15:14(47):43-5 [PubMed PMID: 11974377]
Codd J. Implementation of a patient-held urinary catheter passport to improve catheter management, by prompting for early removal and enhancing patient compliance. Journal of infection prevention. 2014 May:15(3):88-92. doi: 10.1177/1757177413512386. Epub 2013 Nov 28 [PubMed PMID: 28989364]