Introduction
Astereognosis is the inability to identify objects by feeling only without input from the visual system. Stereognosis (from the Greek for "solid" ["stereo"] and "knowledge" ["gnosis"]) is the ability to know the 3-dimensional form of an object with tactile manipulation.[1] Specifically, an object's shape, texture, size, and weight are assessed, usually with the hands. Manual stereognosis requires the dorsal column-medial lemniscus tract (DCMLT) to receive discriminative touch and proprioceptive information and the parietal cortex to process the information. Oral stereognosis is the ability to identify objects by mouth.[2] This is done using the perception of various receptors in the oral structures, including teeth.
Astereognosis, also known as somatosensory agnosia, may also be defined as the impairment of object recognition by somatosensory discrimination of the size, texture, weight, and shape of the objects without any major somatosensory deficit.[3] Postcentral parietal lesions produce astereognosis clinically.[3][4] This is a marker of cognitive impairment in dementia.[5] Astereognosis can be divided into primary and secondary recognition defects.
The primary recognition deficit, called morphognosia, is an impairment in the recognition of the physical features of the object. The secondary recognition deficit is a specific impairment in object recognition with spared primary recognition. The patient can feel the object and sense its dimensions and texture but is unable to correlate with the stored information and identify it.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
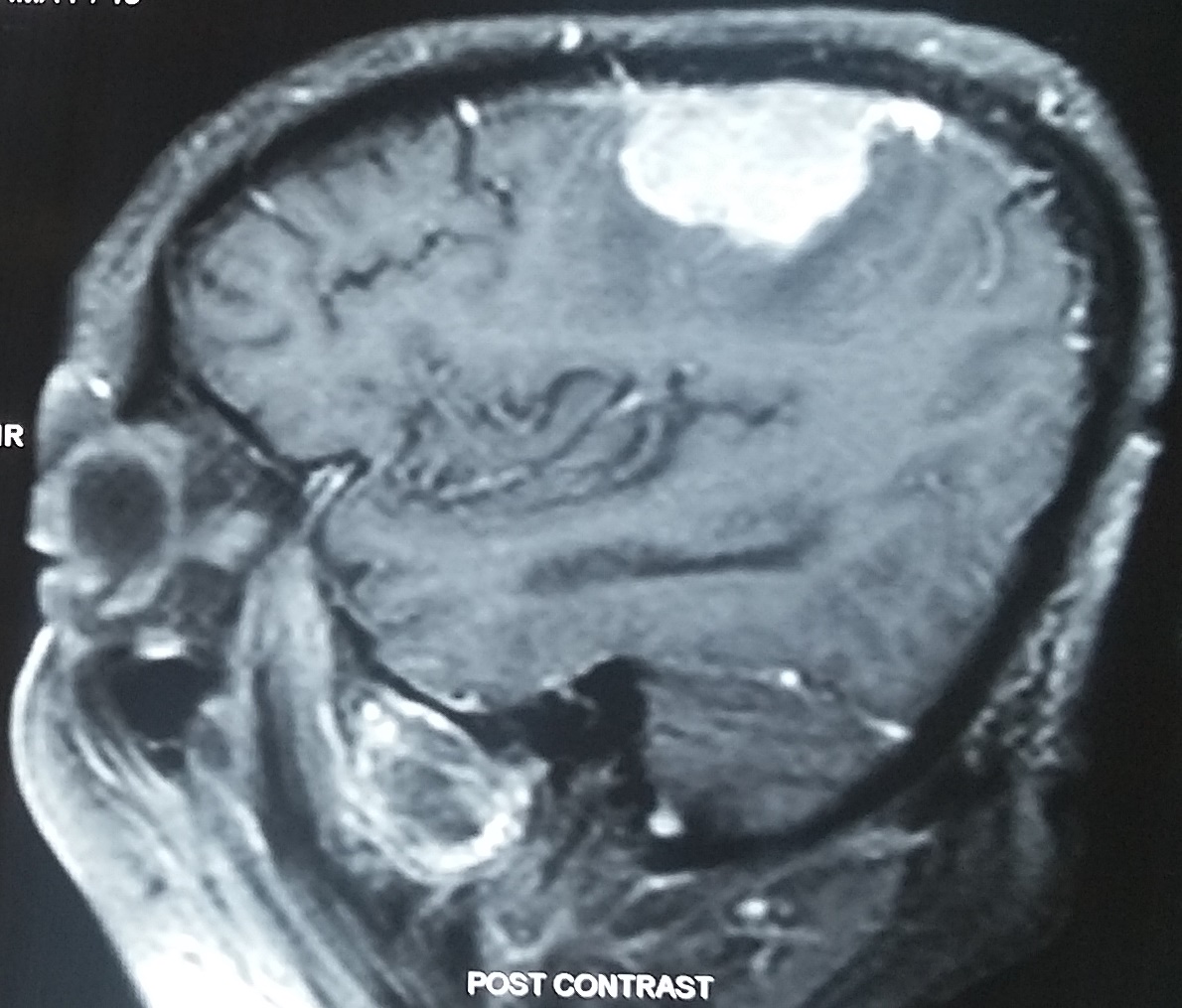
Astereognosis indicates a lesion of the contralateral parietal lobe (see Image. Parietal Meningioma Causing Contralateral Sensory Syndrome). Small postcentral lesions can produce astereognosis, whereas complete parietal lesions produce hemianesthesia on the contralateral side.[4] Bilateral astereognosis can occur with a left postcentral lesion if there is dominance for stereognosis in the left hemisphere. Stroke and neoplasms are common causes. Astereognosis is also seen in diseases with cognitive impairment, such as Alzheimer's disease.[5] Trauma to the parietal regions, such as depressed fracture, also has been reported to cause this.
Other etiologies include ischemic infarction of the parietal lobe.[6] Arteriovenous malformation in the same site can also cause tactile agnosia. Rarely have lesions of the anterior corpus callosum and thalamic radiations been reported to cause astereognosis. Brainstem tumors also cause unilateral astereognosis.[7] Brainstem ischemic lesions involving the medial lemniscus are also causative.[8] It has also been reported in extramedullary tumors of the foramen magnum. High posterior cord lesions due to multiple sclerosis also produce impaired stereognosis.[9]
Astereognosis is associated with the severity of stroke.[10] Somatosensory impairment is associated with motor impairment.[11] A defect in stereognosis is also found in children with cerebral palsy.[12]
Epidemiology
On analysis of the lateralized vascular, neoplastic, or traumatic cerebral lesions for their effect on sensory functions, it was found that cerebrovascular accidents caused severe impairment.[13] The global incidence of stroke is increasing and is around 258 per 100,000 patients a year.[14] Although primary prevention reduces the incidence of stroke in high-income countries, the incidence is increasing in middle-to-low-income countries. The survivors exhibit many impairments. The incidence of brain tumors has increased over the past 40 years.[15] However, this is probably secondary to the increased availability of imaging modalities. The established risk factors for brain tumors are ionizing radiation, mutations of penetrant genes, hereditary syndromes, and immunosuppression.[16] Around 14% of gliomas occur in the parietal lobes.[17] Head injuries can cause astereognosis. The parietal lobe is a common site of depressed skull fracture.[18]
Pathophysiology
Octave Landry formulated the fundamental concepts in the physiology of sensation as proprioception and stereognosis.[19] Manual stereognosis requires the DCMLT to receive discriminative touch and proprioceptive information and the parietal lobe to process the information.[1] The movement of mechanoreceptors relative to one another helps to perceive the 3-dimensional structure of objects.[20]
There are 4 types of mechanoreceptors:
- Merkel cell receptors respond to slowly moving stimuli
- Ruffini corpuscles responding to skin stretch
- Meissner corpuscles for low-frequency vibrations
- Pacinian corpuscles for high-frequency vibrations
They perceive information about the objects' size, shape, texture, and motion. The spatial pattern of the activation of the mechanoreceptor in response to the forces applied to the skin and the relative receptive field of each receptor determine the perception. The information from the forelimb is carried in the dorsal column-medial lemniscal pathway to the cuneate nucleus in the medulla and from there to the ventroposterior lateral nucleus of the thalamus. From the thalamus, the inputs go to the primary somatosensory cortex, Brodmann areas 3, 1, and 2. The information is then carried to the association areas in the posterior parietal cortex and the second somatosensory cortex.
Astereognosis occurs in cortical sensory syndrome secondary to superior-posterior parietal stroke.[21] The parietal cortex is also involved in Alzheimer's disease and the medial temporal lobe, resulting in cognitive impairment.[22] The cortical sensory syndrome is seen in parietal gliomas regardless of hemispheric dominance.[23]
History and Physical
If the tests of peripheral sensations such as light touch, pain, temperature, and vibration are normal, the cortical sensory function can be assessed.[1] The tactile object recognition (TOR) test is done for stereognosis. A series of common objects, such as a pen, key, comb, and paperclip, is placed in the hands of the patient after the eyes are closed. If the patient can recognize the object, stereognosis is intact. In the Nottingham sensory assessment, the light touch test is with cotton wool, pressure by deforming the skin with the index finger, pinprick with a neurotic, and temperature with test tubes filled with hot and cold water.
In the assessment for stereognosis, an object such as a coin, pencil, scissors, cup, glass, comb, or sponge is placed in the hands of the patient for 30 seconds. The patient is asked to identify by naming or description. The score is 2 for normalcy, 1 for impairment (identification of some features of the object), and 0 for absence. The Nottingham method of stereognosis assessment is a reliable tool for raters in patients who have had a stroke.[24]
The sensory deficits in cortical sensory syndrome are loss of position sense, inability to localize noxious stimulus, astereognosis, agraphesthesia, and loss of 2-point discrimination. This is seen contralaterally in parietal lobe disease, hemiparesis, hemianopia, hemineglect, and loss of optokinetic nystagmus. Additional features in the involvement of the dominant parietal lobe will be aphasia, ideomotor apraxia, and Gerstmann syndrome consisting of agraphia without alexia, left-right confusion, digit agnosia, and acalculia.[25] Bilateral astereognosis can occur in dominant parietal lobe dysfunction. The clinical features peculiar to nondominant parietal lobe disease are loss of topographic memory, anosognosia, and dressing apraxia.
Evaluation
Computerized tomography (CT) and magnetic resonance imaging (MRI) are the imaging modalities to assess intracerebral pathology. CT is often the initial imaging in emergencies as it is quick, has no contraindications, and can identify hemorrhage. The use of MRI in acute stroke is increasing.[26] MRI has more sensitivity in acute stroke. In the case of brain tumors, CT may be the initial investigation done for the detection.[27] CT may show calcification, hemorrhage, herniation, mass effect, and hydrocephalus associated with tumors. However, MRI is more useful in characterizing the tumors. Contrast-enhanced T1 weighted images can show the vascularity and necrosis of the tumor. MR spectroscopy will show the metabolic profile. Functional MRI (fMRI) assesses the activities of parts of the brain by detecting the changes in the blood oxygen level-dependent (BOLD) signals. It is widely used to determine the relationship of the tumor to the eloquent areas of the brain.[28]
A study of the somatosensory network after stroke, using resting-state fMRI, has shown strong associations between the interhemispheric network connectivity indices and stereognosis.[29] The higher functional network connectivity is related to better somatosensory function. fMRI has been used to study the primary and secondary somatosensory areas of patients with congenital hemiplegia.[30] Diffusion tensor imaging (DTI) can assess the sensory tract connectivity of the dorsal column medial lemniscus pathway in children with hemiparesis.[31]
Somatosensory evoked potentials (SEP) help correlate sensory impairments such as astereognosis and hemianesthesia with parietal lesions seen in imaging.[4] SEP can also be used as a diagnostic test.
Treatment / Management
The treatment of astereognosis is directed towards the underlying etiology. Treatment of acute ischemic stroke includes intravenous and intraarterial thrombolysis, mechanical thrombectomy, control of blood pressure, antiplatelet drugs, statins, etc.[32] Treatment of neoplasm of the brain may include a combination of surgery, radiotherapy, or chemotherapy, depending on the location, tumor type, and the age of the patient.[33]
Cognitive rehabilitation therapy (CRT) may improve cognitive deficits due to brain trauma, stroke, brain tumor, and dementia.[34][35] Sensory training can improve sensory discrimination after a stroke.[36] Active sensory training consists of manual exploration and discrimination of different surfaces, textures, figures, shapes, weights, and objects with the hands, as well as tactile object recognition.[37] Study of the Effectiveness of Neurorehabilitation on Sensation (SENSe), a randomized controlled trial, showed that sensory discrimination training could improve functional in those with impaired tactile object recognition after stroke.[38](A1)
Differential Diagnosis
There are 3 main syndromes in the differential diagnosis of hemisensory disturbances in a parietal lesion:
- Cortical sensory syndrome: Includes astereognosis, agraphesthesia, and loss of position sense; due to a superior-posterior parietal lesion
- Pseudothalamic sensory syndrome: A faciobrachiocrural impairment of touch, pain, temperature, and vibration; due to an inferior-anterior parietal lesion
- Atypical sensory syndrome: A sensory loss involving all modalities of sensation in a partial distribution; due to parietal lesions of different topography [21]
Astereognosis is used to describe both the inability to discriminate shape and size by touch and the inability to recognize objects by touch. These are apperceptive and associative types of agnosia. The term tactile agnosia is used for the associative type. The deficits in discrimination are most commonly seen with lesions in the primary somatosensory area and its connections. Lesions in the parietal somatosensory association areas result in tactile agnosia.
Tumors at the craniovertebral junction can also produce astereognosis with the involvement of the dorsal column medial lemniscus tract.[39] In addition, amyotrophy of hand muscles may also be seen. Impairment of stereognosis due to lesions in the spinal cord and brainstem is termed stereoanesthesia.[40] Impairment of ipsilateral vibration and joint position senses are noted.
Prognosis
Astereognosis is a frequent somatosensory impairment after a stroke.[10] Recovery occurs over time. Patients with stroke improve over days to months, depending on the location and severity of the stroke.[41] Larger parietal gliomas are associated with neurological deficits.[23] Either improvement or deterioration of the deficit can occur after resection. Patients with traumatic brain injury show functional improvement with rehabilitation therapy, such as sensory re-education, even in the chronic phase.[42]
Complications
Somatosensory loss has a negative influence on functional outcomes. Discriminative sensory impairment of the upper limb is seen in about half of the patients with stroke in the phase of rehabilitation. The sensory impairment of the upper limb results in a hindrance to its usage in daily life.[37] It has been found that impairment of discriminative sensation is common in patients with stroke and causes clumsiness, which is not explained by the main deficits.[43] Astereognosis is associated with a neurocognitive decline in Alzheimer's disease.[5] Astereognosis or stereoanesthesia is associated with useless hand syndrome or numb, clumsy hands in high cervical lesions due to multiple sclerosis or cervical spondylosis.[9][44][45] Impaired stereognosis in parietal lobe disease can lead to tactile apraxia, as a disturbance of hand movements for interaction with an object.[46]
Deterrence and Patient Education
Patients are given sensory re-education to improve the deficits. This is by giving graded tactile stimuli to restore sensory areas.[37] Sensory re-learning comprises the tactile exploration of surfaces, tactile discrimination of shapes, textures, weights, and temperatures, and tactile object recognition of objects. The patients are asked to do repeated exercises with grades of increasing difficulty. The feedback is taken with vision or by the unaffected hand. Individualized home exercises are also advised.
Enhancing Healthcare Team Outcomes
Healthcare professionals should be trained to elicit discriminatory and cortical sensations. The Nottingham sensory assessment is a good method to assess tactile and kinesthetic sensations and stereognosis. Patients with astereognosis are best managed with an interprofessional team approach. There is a significant role in rehabilitation after the acute treatment of neurological diseases. This may include neurologists, physiatrists, occupational therapy, physical therapy, and speech therapy. Sensory training should be given as much importance as motor training. Therapists' early involvement in the disease is important for optimal outcomes. The competence and training of physical and occupational therapists in astereognosis are vital.
Media
(Click Image to Enlarge)
References
Schermann T, Tadi P. Stereognosis. StatPearls. 2024 Jan:(): [PubMed PMID: 32310463]
Jacobs R, Bou Serhal C, van Steenberghe D. Oral stereognosis: a review of the literature. Clinical oral investigations. 1998 Mar:2(1):3-10 [PubMed PMID: 9667147]
Roland PE. Astereognosis. Tactile discrimination after localized hemispheric lesions in man. Archives of neurology. 1976 Aug:33(8):543-50 [PubMed PMID: 942311]
Mauguière F, Desmedt JE, Courjon J. Astereognosis and dissociated loss of frontal or parietal components of somatosensory evoked potentials in hemispheric lesions. Detailed correlations with clinical signs and computerized tomographic scanning. Brain : a journal of neurology. 1983 Jun:106 (Pt 2)():271-311 [PubMed PMID: 6850271]
Level 3 (low-level) evidenceDavis AS, Mazur-Mosiewicz A, Dean RS. The presence and predictive value of astereognosis and agraphesthesia in patients with Alzheimer's disease. Applied neuropsychology. 2010 Oct:17(4):262-6. doi: 10.1080/09084282.2010.525102. Epub [PubMed PMID: 21154039]
Hömke L, Amunts K, Bönig L, Fretz C, Binkofski F, Zilles K, Weder B. Analysis of lesions in patients with unilateral tactile agnosia using cytoarchitectonic probabilistic maps. Human brain mapping. 2009 May:30(5):1444-56. doi: 10.1002/hbm.20617. Epub [PubMed PMID: 18636551]
Level 3 (low-level) evidenceFeinsod M, Bentin S, Moscovitch M, Wald U. Brainstem tumor presenting with unilateral astereognosis. Annals of neurology. 1980 Aug:8(2):191-2 [PubMed PMID: 7425573]
Level 3 (low-level) evidenceEndtz LJ, Frenay JJ. Studies on asterognosis and amyotrophy of the hand in brainstem syndromes. Relation to the symptomatology of tumours at the spinocranial junction. Journal of the neurological sciences. 1980 Jan:44(2-3):241-6 [PubMed PMID: 7354369]
Kamogawa K, Okuda B. Useless hand syndrome with astereognosis in multiple sclerosis. Multiple sclerosis and related disorders. 2015 Jan:4(1):85-7. doi: 10.1016/j.msard.2014.09.212. Epub 2014 Oct 7 [PubMed PMID: 25787059]
Level 3 (low-level) evidenceConnell LA, Lincoln NB, Radford KA. Somatosensory impairment after stroke: frequency of different deficits and their recovery. Clinical rehabilitation. 2008 Aug:22(8):758-67. doi: 10.1177/0269215508090674. Epub [PubMed PMID: 18678576]
Meyer S, De Bruyn N, Krumlinde-Sundholm L, Peeters A, Feys H, Thijs V, Verheyden G. Associations Between Sensorimotor Impairments in the Upper Limb at 1 Week and 6 Months After Stroke. Journal of neurologic physical therapy : JNPT. 2016 Jul:40(3):186-95. doi: 10.1097/NPT.0000000000000138. Epub [PubMed PMID: 27214520]
Van Heest AE, House J, Putnam M. Sensibility deficiencies in the hands of children with spastic hemiplegia. The Journal of hand surgery. 1993 Mar:18(2):278-81 [PubMed PMID: 8463594]
Hom J, Reitan RM. Effect of lateralized cerebral damage upon contralateral and ipsilateral sensorimotor performances. Journal of clinical neuropsychology. 1982 Sep:4(3):249-68 [PubMed PMID: 7142422]
Béjot Y, Daubail B, Giroud M. Epidemiology of stroke and transient ischemic attacks: Current knowledge and perspectives. Revue neurologique. 2016 Jan:172(1):59-68. doi: 10.1016/j.neurol.2015.07.013. Epub 2015 Dec 21 [PubMed PMID: 26718592]
Level 3 (low-level) evidenceFisher JL, Schwartzbaum JA, Wrensch M, Wiemels JL. Epidemiology of brain tumors. Neurologic clinics. 2007 Nov:25(4):867-90, vii [PubMed PMID: 17964019]
Wrensch M, Minn Y, Chew T, Bondy M, Berger MS. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro-oncology. 2002 Oct:4(4):278-99. doi: 10.1093/neuonc/4.4.278. Epub [PubMed PMID: 12356358]
Larjavaara S, Mäntylä R, Salminen T, Haapasalo H, Raitanen J, Jääskeläinen J, Auvinen A. Incidence of gliomas by anatomic location. Neuro-oncology. 2007 Jul:9(3):319-25 [PubMed PMID: 17522333]
Satardey RS, Balasubramaniam S, Pandya JS, Mahey RC. Analysis of Factors Influencing Outcome of Depressed Fracture of Skull. Asian journal of neurosurgery. 2018 Apr-Jun:13(2):341-347. doi: 10.4103/ajns.AJNS_117_16. Epub [PubMed PMID: 29682032]
Walusinski O. Pioneering the concepts of stereognosis and polyradiculoneuritis: Octave Landry (1826-1865). European neurology. 2013:70(5-6):281-90. doi: 10.1159/000353167. Epub 2013 Sep 17 [PubMed PMID: 24051983]
Yau JM, Kim SS, Thakur PH, Bensmaia SJ. Feeling form: the neural basis of haptic shape perception. Journal of neurophysiology. 2016 Feb 1:115(2):631-42. doi: 10.1152/jn.00598.2015. Epub 2015 Nov 18 [PubMed PMID: 26581869]
Bassetti C, Bogousslavsky J, Regli F. Sensory syndromes in parietal stroke. Neurology. 1993 Oct:43(10):1942-9 [PubMed PMID: 8413950]
Jacobs HI, Van Boxtel MP, Jolles J, Verhey FR, Uylings HB. Parietal cortex matters in Alzheimer's disease: an overview of structural, functional and metabolic findings. Neuroscience and biobehavioral reviews. 2012 Jan:36(1):297-309. doi: 10.1016/j.neubiorev.2011.06.009. Epub 2011 Jun 30 [PubMed PMID: 21741401]
Level 2 (mid-level) evidenceRussell SM, Elliott R, Forshaw D, Kelly PJ, Golfinos JG. Resection of parietal lobe gliomas: incidence and evolution of neurological deficits in 28 consecutive patients correlated to the location and morphological characteristics of the tumor. Journal of neurosurgery. 2005 Dec:103(6):1010-7 [PubMed PMID: 16381187]
Level 3 (low-level) evidenceGaubert CS, Mockett SP. Inter-rater reliability of the Nottingham method of stereognosis assessment. Clinical rehabilitation. 2000 Apr:14(2):153-9 [PubMed PMID: 10763792]
Altabakhi IW, Liang JW. Gerstmann Syndrome. StatPearls. 2024 Jan:(): [PubMed PMID: 30137813]
Chalela JA, Kidwell CS, Nentwich LM, Luby M, Butman JA, Demchuk AM, Hill MD, Patronas N, Latour L, Warach S. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet (London, England). 2007 Jan 27:369(9558):293-8 [PubMed PMID: 17258669]
Mabray MC, Barajas RF Jr, Cha S. Modern brain tumor imaging. Brain tumor research and treatment. 2015 Apr:3(1):8-23. doi: 10.14791/btrt.2015.3.1.8. Epub 2015 Apr 29 [PubMed PMID: 25977902]
Vlieger EJ, Majoie CB, Leenstra S, Den Heeten GJ. Functional magnetic resonance imaging for neurosurgical planning in neurooncology. European radiology. 2004 Jul:14(7):1143-53 [PubMed PMID: 15148622]
De Bruyn N, Meyer S, Kessner SS, Essers B, Cheng B, Thomalla G, Peeters A, Sunaert S, Duprez T, Thijs V, Feys H, Alaerts K, Verheyden G. Functional network connectivity is altered in patients with upper limb somatosensory impairments in the acute phase post stroke: A cross-sectional study. PloS one. 2018:13(10):e0205693. doi: 10.1371/journal.pone.0205693. Epub 2018 Oct 12 [PubMed PMID: 30312350]
Level 2 (mid-level) evidenceFiori S, Biagi L, Cecchi P, Cioni G, Beani E, Tosetti M, Cosottini M, Guzzetta A. Potentials of Ultrahigh-Field MRI for the Study of Somatosensory Reorganization in Congenital Hemiplegia. Neural plasticity. 2018:2018():8472807. doi: 10.1155/2018/8472807. Epub 2018 Nov 25 [PubMed PMID: 30595689]
Kuczynski AM, Carlson HL, Lebel C, Hodge JA, Dukelow SP, Semrau JA, Kirton A. Sensory tractography and robot-quantified proprioception in hemiparetic children with perinatal stroke. Human brain mapping. 2017 May:38(5):2424-2440. doi: 10.1002/hbm.23530. Epub 2017 Feb 8 [PubMed PMID: 28176425]
Hui C, Tadi P, Khan Suheb MZ, Patti L. Ischemic Stroke. StatPearls. 2024 Jan:(): [PubMed PMID: 29763173]
Perkins A, Liu G. Primary Brain Tumors in Adults: Diagnosis and Treatment. American family physician. 2016 Feb 1:93(3):211-7 [PubMed PMID: 26926614]
Laatsch L, Dodd J, Brown T, Ciccia A, Connor F, Davis K, Doherty M, Linden M, Locascio G, Lundine J, Murphy S, Nagele D, Niemeier J, Politis A, Rode C, Slomine B, Smetana R, Yaeger L. Evidence-based systematic review of cognitive rehabilitation, emotional, and family treatment studies for children with acquired brain injury literature: From 2006 to 2017. Neuropsychological rehabilitation. 2020 Jan:30(1):130-161. doi: 10.1080/09602011.2019.1678490. Epub 2019 Oct 31 [PubMed PMID: 31671014]
Level 1 (high-level) evidenceCicerone KD, Dahlberg C, Kalmar K, Langenbahn DM, Malec JF, Bergquist TF, Felicetti T, Giacino JT, Harley JP, Harrington DE, Herzog J, Kneipp S, Laatsch L, Morse PA. Evidence-based cognitive rehabilitation: recommendations for clinical practice. Archives of physical medicine and rehabilitation. 2000 Dec:81(12):1596-615 [PubMed PMID: 11128897]
Byl N, Roderick J, Mohamed O, Hanny M, Kotler J, Smith A, Tang M, Abrams G. Effectiveness of sensory and motor rehabilitation of the upper limb following the principles of neuroplasticity: patients stable poststroke. Neurorehabilitation and neural repair. 2003 Sep:17(3):176-91 [PubMed PMID: 14503438]
Level 1 (high-level) evidenceCarlsson H, Rosén B, Pessah-Rasmussen H, Björkman A, Brogårdh C. SENSory re-learning of the UPPer limb after stroke (SENSUPP): study protocol for a pilot randomized controlled trial. Trials. 2018 Apr 17:19(1):229. doi: 10.1186/s13063-018-2628-1. Epub 2018 Apr 17 [PubMed PMID: 29665842]
Level 3 (low-level) evidenceCarey L, Macdonell R, Matyas TA. SENSe: Study of the Effectiveness of Neurorehabilitation on Sensation: a randomized controlled trial. Neurorehabilitation and neural repair. 2011 May:25(4):304-13. doi: 10.1177/1545968310397705. Epub 2011 Feb 24 [PubMed PMID: 21350049]
Level 1 (high-level) evidenceFrenay JJ, Groen JJ, Endtz LJ. Tumours at the spinocranial junction: some clinical and electromyographic aspects in relation to the symptomatology. Clinical neurology and neurosurgery. 1979:81(1):13-25 [PubMed PMID: 223793]
Level 3 (low-level) evidenceKararizou E, Lykomanos D, Kosma A, Kokotis P, Giatas K, Markou I, Vassilopoulos D. Stereoanesthesia or astereognosia? Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2009 Oct:30(5):409-11. doi: 10.1007/s10072-009-0117-8. Epub 2009 Jul 8 [PubMed PMID: 19585078]
Level 3 (low-level) evidenceLee KB, Lim SH, Kim KH, Kim KJ, Kim YR, Chang WN, Yeom JW, Kim YD, Hwang BY. Six-month functional recovery of stroke patients: a multi-time-point study. International journal of rehabilitation research. Internationale Zeitschrift fur Rehabilitationsforschung. Revue internationale de recherches de readaptation. 2015 Jun:38(2):173-80. doi: 10.1097/MRR.0000000000000108. Epub [PubMed PMID: 25603539]
Gupta A, Taly AB. Functional outcome following rehabilitation in chronic severe traumatic brain injury patients: A prospective study. Annals of Indian Academy of Neurology. 2012 Apr:15(2):120-4. doi: 10.4103/0972-2327.94995. Epub [PubMed PMID: 22566725]
Kim JS, Choi-Kwon S. Discriminative sensory dysfunction after unilateral stroke. Stroke. 1996 Apr:27(4):677-82 [PubMed PMID: 8614929]
Hamada E, Okamoto K, Okuda B. [A case of multiple sclerosis with bilateral useless hand syndrome as a main clinical feature]. Rinsho shinkeigaku = Clinical neurology. 2005 Mar:45(3):211-5 [PubMed PMID: 15835290]
Level 3 (low-level) evidenceGood DC, Couch JR, Wacaser L. "Numb, clumsy hands" and high cervical spondylosis. Surgical neurology. 1984 Sep:22(3):285-91 [PubMed PMID: 6463840]
Level 3 (low-level) evidenceBinkofski F, Kunesch E, Classen J, Seitz RJ, Freund HJ. Tactile apraxia: unimodal apractic disorder of tactile object exploration associated with parietal lobe lesions. Brain : a journal of neurology. 2001 Jan:124(Pt 1):132-44 [PubMed PMID: 11133793]
Level 1 (high-level) evidence