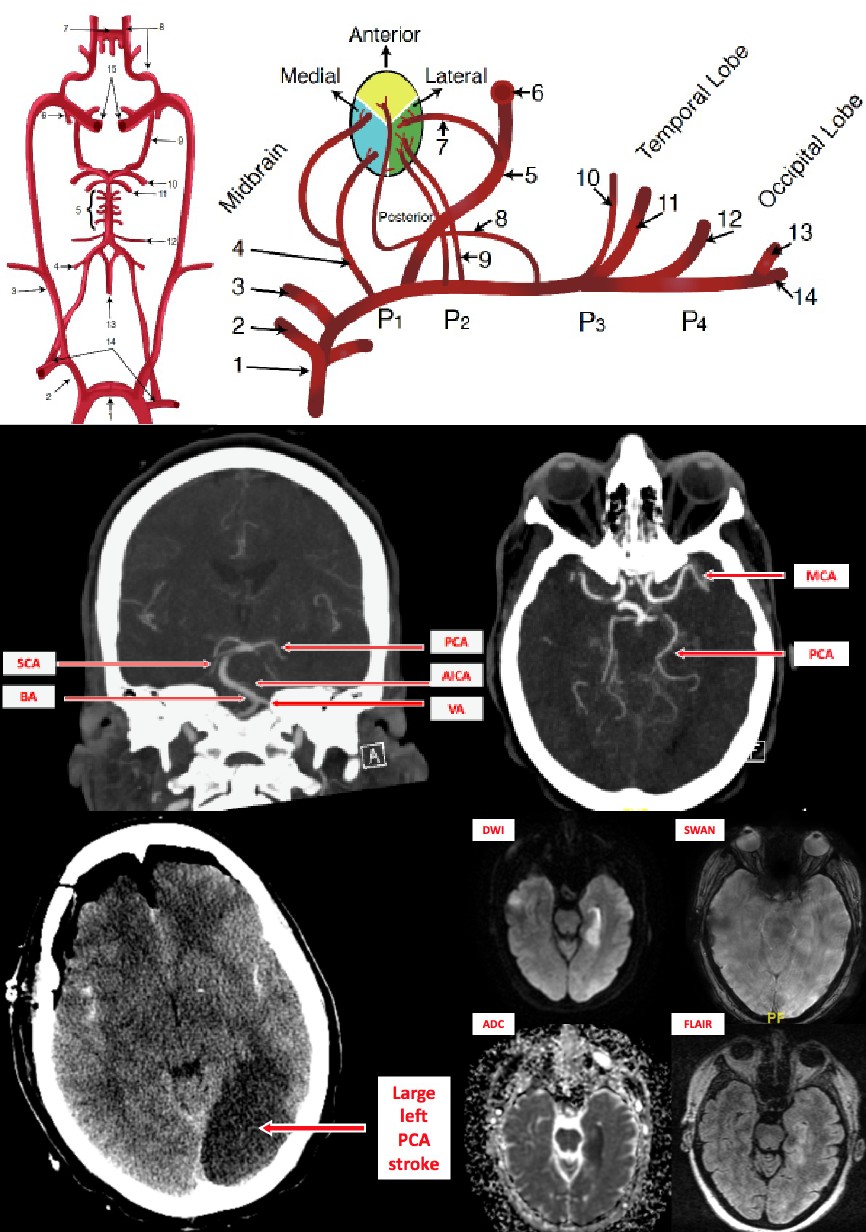
Introduction
Acute stroke is frequently referred to as a cerebrovascular accident; however, it is essential to note that a stroke is not an accidental event. A more accurate and meaningful term to describe it is "brain attack," which carries a similar significance to "heart attack." However, stroke encompasses a broader range of variations than heart disease. Stroke is categorized into mainly 2 types: ischemic and hemorrhagic. Hemorrhagic strokes are further divided into intracerebral hemorrhage (ICH) and subarachnoid hemorrhage (SAH), more specifically, nontraumatic (spontaneous) ICH and nontraumatic (spontaneous aneurysmal) SAH.[1]
Ischemic strokes occur when there is a blockage in a blood vessel, resulting in a restricted blood supply to the brain. In contrast, hemorrhagic strokes occur when a blood vessel ruptures, causing blood to leak into the intracranial cavity.
The American Heart Association/The American Stroke Association (AHA/ASA) provides a comprehensive definition of stroke.[1] In its simplest form, stroke is an acute episode of focal neurological dysfunction that persists for more than 24 hours.
Stroke ranks as the second leading cause of death worldwide and is a major contributor to disability.[2][3][4] Stroke imposes a considerable financial burden due to the costs associated with prehospital, hospital, and posthospital care.[5][6][7]
Understanding that the potential for achieving a complete neurological recovery diminishes with every minute of untreated acute stroke is essential. This forms the "time is brain" concept foundation, emphasizing the critical importance of timely evaluation and management of an acute stroke. Early and targeted treatments, rehabilitation programs, and long-term lifestyle modifications can significantly enhance clinical outcomes for individuals with an acute stroke. This can hopefully lead to maximal clinical recovery in each patient and decrease the global impact of stroke.[8]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Ischemic Stroke
Ischemic stroke exhibits significant heterogeneity, with more than 100 implicated pathologies.[9] The Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification system identifies 3 primary causes of ischemic stroke: large vessel disease, small vessel disease (lacunar), and cardioembolism.[10]
Large vessel disease encompasses conditions such as atherosclerosis, arterial dissection, and artery-to-artery embolism. When major arteries experience thrombotic or embolic occlusion, specific syndromes can manifest due to reduced blood flow to particular brain regions, which correlates with corresponding examination findings. Large vessels comprise intracranial arteries (including the circle of Willis and its proximal branches) and extracranial arteries (including common carotid, internal carotid, and vertebral arteries).[11][12]
Lacunar strokes, predominantly caused by small vessel diseases, are commonly associated with lipohyalinosis and atherosclerosis. Lipohyalinosis refers to the concentric hyaline thickening of small cerebral vessels leading to the occlusion of penetrating arteries. Atherosclerotic plaques in parent arteries, particularly involving the ostium of perforating branches, can also result in occlusion. Furthermore, microatheromas have the potential to obstruct small penetrating arteries.[13][14]
Cardioembolism, as a cause of stroke, can stem from various sources, including arrhythmia, valvular heart disease, bioprosthetic and mechanical heart valves, and cardiomyopathy.[10][11][12]
Ischemic stroke is primarily associated with several key risk factors, including advanced age, hypertension, diabetes, hyperlipidemia, cigarette smoking, arrhythmia, and cardiac disease.[11][12][14]
Intracerebral Hemorrhage (ICH)
ICH stands as the second most prevalent form of stroke. ICH is typically caused by the rupture of small arteries, often due to hypertensive vasculopathy, cerebral amyloid angiopathy (CAA), coagulopathies, and other vasculopathies. Hypertensive vasculopathy is predominantly associated with non-lobar ICH (in regions such as the basal ganglia, thalamus, cerebellum, and brainstem), whereas CAA is more commonly linked to lobar ICH.[15] Several risk factors contribute to ICH, including advancing age, hypertension, CAA, smoking, excessive alcohol intake, sympathomimetic drugs, anticoagulants, and antiplatelet drugs.[16][17]
Subarachnoid Hemorrhage (SAH)
Approximately 5% of all strokes are caused by spontaneous SAH due to a ruptured aneurysm in 85% of patients. Other causes of spontaneous SAH include drug use (such as amphetamines and cocaine), coagulopathy, a ruptured arteriovenous malformation, and vessel rupture due to a dural venous sinus thrombosis. Various risk factors are associated with SAH, including smoking, hypertension, excessive alcohol consumption, advancing age, personal history of another type of aneurysm or SAH, and family history of an intracranial aneurysm.[18][19][20][21]
Epidemiology
Stroke ranks as the second leading cause of death worldwide and is a major contributor to disability. Ischemic strokes account for approximately 62% of all strokes, followed by ICH at 28% and SAH at 10%.[2][3][4] Although ischemic strokes are more prevalent, hemorrhagic strokes result in more fatalities and lost disability-adjusted life-years (DALYs).[3]
Between 1990 and 2019, ICH and SAH demonstrated more significant reductions worldwide in age-standardized rates per year, compared to ischemic stroke, for incident and prevalent strokes, deaths resulting from stroke, and DALYs due to stroke.[22]
Both men and women worldwide face an approximate lifetime risk of stroke of 25% starting from age 25. The risk is notably high in East Asia and Central and Eastern Europe.[23]
Pathophysiology
Ischemic Stroke
The pathophysiology of ischemic stroke begins with insufficient blood supply to a focal area of brain tissue. Within minutes, the central core of tissue in this affected area progresses toward irreversible damage, known as the area of infarction. However, the surrounding tissue, referred to as the penumbra, does not experience immediate cell death and has the potential for recovery if early reperfusion is achieved.[24]
In regions of reduced blood flow, there is an imbalance between the consumption and production of adenosine triphosphate (ATP), resulting in diminished energy stores. This leads to ionic imbalances, electrical disturbances, and a cascade of ischemia-related changes. These changes increase the production of reactive oxygen species (ROS) and nitric oxide (NO). Over time, the pathophysiological cascade destroys cell membranes, cell lysis, and cell death through mechanisms such as necrosis or apoptosis.[25]
Following ischemic stroke, microglia are swiftly activated in the affected ischemic area and extend to the penumbra region. Their activation peaks 48 to 72 hours after the stroke onset and can persist for several weeks.[26][27] Activated microglia cause an increase in proinflammatory cytokines such as ROS, NO, interleukin-1β, and tumor necrosis factor-α. However, they also release anti-inflammatory cytokines and neurotrophic factors, including brain-derived neurotrophic factor, glial cell-line-derived neurotrophic factor, and basic fibroblast growth factor.[28][27][29][30][31]
The intricate ischemic cascade triggered by acute stroke ultimately leads to the loss of neurons and supporting structures.
Intracerebral Hemorrhage (ICH)
After the rupture of small arteries due to hypertensive changes, CAA, coagulopathies, and other vasculopathies, the primary injury mechanisms in ICH involve the expanding hematoma's mass effect and perihematomal edema. The growing hematoma volume and edema contribute to elevated intracranial pressure (ICP), potentially reducing cerebral perfusion and ischemic injury. In addition, patients are also at risk of experiencing intraventricular hemorrhage (IVH) and herniation.[32][16][17]
Similar to ischemic stroke, ICH also undergoes subsequent proinflammatory and anti-inflammatory phases. Secondary mechanisms of injury (such as blood-related cytotoxicity, excitotoxicity, and oxidative stress) disrupt the blood-brain barrier, resulting in significant brain cell death and the development of potentially life-threatening brain edema.[32][33]
Subarachnoid Hemorrhage (SAH)
A cerebral aneurysm, when ruptured, is the primary cause of SAH. However, it's noteworthy that brain injury arising from a cerebral aneurysm can occur without an actual rupture. Compressive forces exerted by the aneurysm can damage local brain tissue and compromise the blood supply to distal areas.
When an aneurysm ruptures, arterial blood is released into the subarachnoid space, rapidly disseminating through the cerebrospinal fluid and increasing ICP. The arterial blood can also extend into the intraventricular space and brain parenchyma.
Secondary brain injury can occur due to several factors, including ICH, IVH, ICP, hydrocephalus, subdural hematoma, or delayed cerebral ischemia (DCI).[34]
History and Physical
A rapid, focused history and physical examination are essential for evaluating a patient presenting with acute-onset focal neurological dysfunction. These initial steps are crucial in differentiating acute stroke from stroke mimics and determining the etiology (whether it is ischemic or hemorrhagic, including the subtype). Although statistics may vary, common ischemic stroke mimics include hypoglycemia, seizure, and migraine.[35]
Ischemic Stroke
Ideally, a patient's history should include information about when they were last known to be well, the understanding of onset, any risk factors, medications, and other relevant details regarding a possible underlying illness. Along with assessing vital signs, conducting a targeted neurological examination using the National Institutes of Health Stroke Scale (NIHSS) is advisable.[12]
Intracerebral Hemorrhage (ICH)
Although ICH most commonly occurs during routine activities, it can also occur during sexual intercourse or other physical activities. Neurological symptoms tend to worsen over minutes to a few hours progressively. During hospitalization, neurological deterioration is frequently observed due to the expansion of the hematoma and its associated consequences. The clinical presentation of ICH varies depending on the size and location of the hemorrhage, with prevalent symptoms including nausea, vomiting, and headache.[16][36]
Seizures typically occur at the onset of bleeding or within 24 hours. The incidence of acute seizures during the first 24 to 72 hours ranges from 4% to 42%.[37][38]
Without neuroimaging, no clinical decision scale can differentiate ICH from other highly sensitive or specific conditions.[39]
Subarachnoid Hemorrhage (SAH)
Similar to ICH, aneurysmal SAH usually occurs during routine activity, including rest or sleep, but it can also occur during physical activity.[40][41] SAH is most commonly characterized by a sudden and intense headache known as a "thunderclap headache," often described as "the most severe or worst headache ever experienced in life." Accompanying symptoms may include neck pain or stiffness, photophobia, vomiting, altered mental status, and loss of consciousness. Some patients may report a sentinel headache days or weeks before presentation. Furthermore, seizures may also occur during hemorrhage, hospitalization, or as a long-term complication of aneurysmal SAH.[34]
Potential focal examination findings include unilateral vision loss, visuospatial neglect, ophthalmoplegia, and retinal, subhyaloid, and vitreous hemorrhage. Other possible findings include third and sixth nerve palsy, hemiparesis, aphasia, and abulia.[42]
The neurological examination can be categorized using the Hunt-Hess scale or the World Federation of Neurological Surgeons scale.[34]
Evaluation
Neuroimaging is a critical component of stroke management, with computed tomography (CT) and magnetic resonance imaging (MRI) being the primary modalities.[43] According to the 2019 AHA/ASA Guidelines for the Management of acute ischemic stroke (AIS), it is recommended that all patients suspected of experiencing acute stroke should undergo emergency brain imaging evaluation upon arrival at the hospital before initiating any specific therapy to treat AIS. Noncontrast CT (NCCT) and MRI are considered appropriate modalities to exclude ICH before administering intravenous (IV) alteplase.[44]
In cases where a patient with AIS presents within 6 hours of symptom onset and shows a small core infarct on NCCT, it is recommended to use CT angiography (CTA) or MR angiography (MRA) for guiding mechanical thrombectomy patient selection. Conversely, when a patient with AIS presents within the 6- to 24-hour window following symptom onset and displays a large-vessel occlusion (LVO) in the anterior circulation, it is recommended to use diffusion-weighted MRI (DW-MRI) with or without MR perfusion or CT perfusion for evaluation.[45][46]
For patients experiencing a "wake-up" stroke or with an uncertain time of symptom onset, obtaining an MRI is crucial in identifying diffusion-positive fluid-attenuated inversion recovery (FLAIR)-negative lesions. This imaging technique assists in determining whether the patient would benefit from thrombolytic therapy.[47]
In patients diagnosed with ICH, performing a CTA within the first few hours of symptom onset can help identify individuals at risk of experiencing hematoma expansion (HE). Furthermore, serial head CT scans within the first 24 hours can assess for any HE.[39]
The diagnosis of SAH is primarily based on NCCT imaging. A lumbar puncture is recommended if the CT scan yields negative results despite a high clinical suspicion. A recent study found that when the Ottawa SAH rule was employed to interpret a CT scan conducted within 6 hours of headache onset, the diagnosis of SAH demonstrated a sensitivity of 95.5% and specificity of 100%.[48]
When assessing SAH, CTA can be taken into consideration. However, if the results of CTA are inconclusive, the gold standard study for detecting aneurysms is digital subtraction catheter angiography with 3-dimensional reconstruction.[49][50]
As per the 2019 AHA/ASA Guidelines, it is recommended to assess the blood glucose levels in all patients before initiating IV alteplase because both hypoglycemia and hyperglycemia can mimic AIS. Furthermore, conducting a baseline electrocardiographic assessment is advised for patients presenting with AIS, but it should not delay the initiation of IV alteplase. Similarly, a baseline troponin assessment is recommended, but it should not hinder the initiation of IV alteplase or mechanical thrombectomy.[44]
Treatment / Management
The chances of complete neurological recovery decrease with every minute an acute stroke remains untreated. This forms the "time is brain" concept foundation, highlighting the importance of promptly evaluating and managing acute stroke cases.
Ischemic Stroke
Here are the key highlights from the 2019 AHA/ASA Management Guidelines for AIS:[44]
- Airway, breathing, and oxygenation: Supplemental oxygen should be administered to patients to maintain the oxygen saturation above 94%. However, it is not recommended for nonhypoxic patients.
- Blood pressure (BP): Patients with elevated BP should have their BP carefully reduced to a systolic BP (SBP) below 185 mm Hg and diastolic BP below 110 mm Hg before initiating IV fibrinolytic therapy. Following the treatment, the BP should be maintained below 180/105 mm Hg for at least 24 hours. Suppose a mechanical thrombectomy is planned, and the patient has not received IV fibrinolytic therapy. In that case, it is reasonable to maintain their BP at or below 185/110 mm Hg before the procedure and at or below 180/105 mm Hg during and for 24 hours after the procedure.
- Temperature: In patients with AIS, hyperthermia (body temperature >38°C or 100.4°F) should be addressed by administering antipyretic medication.
- Blood glucose: Both hypoglycemia (blood glucose level <60 mg/dL) and hyperglycemia (blood glucose levels within the range of 140 to 180 mg/dL) should be treated in patients with AIS.
- IV alteplase: IV alteplase is recommended for patients with AIS at a dosage of 0.9 mg/kg, with the initial 10% given as a bolus over 1 minute (maximum dosage is 90 mg over 60 minutes). Eligible patients include those who meet the criteria within 3 hours or 3 to 4.5 hours of AIS witnessed symptom onset or when the patient was last known well or at baseline. The exact dosage can be administered within 4.5 hours to patients with AIS who awaken with stroke symptoms or have an unclear time of onset greater than 4.5 hours from when they were last known well or at baseline, and also for patients who have a DW-MRI lesion smaller than one-third of the middle cerebral artery (MCA) territory and no visible signal change on FLAIR. To mitigate the risk of bleeding, abciximab should not be administered concurrently with IV alteplase; IV aspirin should not be administered within 90 minutes after initiating IV alteplase; and IV alteplase should not be administered within 24 hours after a total treatment dosage of low-molecular-weight heparin.
- Other IV fibrinolytics: In patients without contraindications who are eligible for mechanical thrombectomy, a single IV bolus of tenecteplase at 0.25 mg/kg (maximum 25 mg) can be administered instead of IV alteplase. However, IV defibrinogenating or IV fibrinolytic agents, other than alteplase and tenecteplase, are not recommended.
- Mechanical thrombectomy: Patients eligible for IV alteplase should receive it, even if mechanical thrombectomy is being considered. However, if a patient fulfills all 6 of the following criteria, they should undergo mechanical thrombectomy using a stent retriever or direct aspiration: (1) pre-stroke modified Rankin Scale score of 0 to 1; (2) AIS caused by an occlusion in the internal carotid artery or MCA segment 1 (M1); (3) age 18 or older; (4) NIHSS score of 6 or higher; (5) Alberta Stroke Program Early CT Score (ASPECTS) of 6 or higher; and (6) treatment can be initiated within 6 hours of symptom onset.
Mechanical thrombectomy is also recommended for patients within 6 to 16 hours of witnessed symptom onset or 16 to 24 hours from the patient's last known well or at baseline. These patients must exhibit LVO in the anterior circulation and meet additional criteria according to the DAWN or DEFUSE 3 trials.[45][51](B3)
- Antiplatelet treatment: Aspirin administration is recommended within 24 to 48 hours after symptom onset. However, aspirin administration is typically delayed until 24 hours after treating a patient with IV alteplase. For patients diagnosed with minor, noncardioembolic ischemic stroke who have not received IV alteplase, it is appropriate to initiate dual antiplatelet therapy with aspirin and clopidogrel within 24 hours after symptom onset.
Intracerebral Hemorrhage
Initiating treatment for elevated BP within 2 hours of ICH onset and achieving the target BP within 1 hour may reduce HE risk and improve outcomes.[39]
In patients with mild-to-moderate ICH and an initial SBP between 150 and 220 mm Hg, the target SBP is 140 mm Hg to maintain the SBP between 130 and 150 mm Hg.[39] It is noteworthy that if these patients present with an initial SBP above 150 mm Hg, rapidly lowering the SBP to below 130 mm Hg may cause potential harm.[39]
Anticoagulation should be discontinued immediately in patients with anticoagulant-associated ICH, and rapid reversal should be performed as soon as possible.[39][52](B3)
Platelet transfusion may be considered in patients treated with aspirin who require emergency neurosurgery.[39][53] However, platelet transfusion should not be administered for patients treated with aspirin who do not require emergency neurosurgery due to potentially harmful effects.[39][54](A1)
Surgical management has reduced mortality for specific patients compared to medical management alone. Surgical options for managing ICH include minimally invasive hematoma evacuation with endoscopic or stereotactic aspiration, external ventricular drain (EVD) insertion, and craniotomy.[39]
Subarachnoid Hemorrhage
According to the latest guidelines from the European Stroke Organization (ESO) and AHA/ASA, early treatment of aneurysms is recommended to decrease the risk of rebleeding. The ESO recommends intervention within 72 hours after symptom onset.[50][55] However, a recent meta-analysis on the timing of endovascular treatment in SAH indicates a lack of evidence regarding the optimal timing in SAH patients.[56](A1)
Short-term antifibrinolytic therapy was considered a strategy to reduce the rebleeding risk; however, the routine use of tranexamic acid after SAH cannot be recommended.[57](A1)
Between the onset of SAH symptoms and the obliteration of the aneurysm, AHA/ASA recommends maintaining SBP below 160 mm Hg. On the contrary, ESO suggests maintaining the SBP below 180 mm Hg until the ruptured aneurysm has been coiled or clipped.[34][50][55] Complete obliteration of the aneurysm is the recommended goal, with endovascular coiling being the preferred treatment when a ruptured aneurysm is considered suitable for either coiling or clipping.[34][50][55]
In case of a patient experiencing a seizure associated with SAH, treatment with antiepileptic drugs is recommended.[55] Short-term seizure prophylaxis may also be considered during the immediate posthemorrhagic period.[50] Depending on the clinical situation, the management of hydrocephalus involves diverting cerebrospinal fluid through methods such as EVD or lumbar drainage.[34][50][55] Nimodipine should be administered to all patients to prevent DCI, with oral administration preferred over IV administration.[34][50][55] Additional treatment goals include pain control, euvolemia, normothermia, and normoglycemia.
Differential Diagnosis
The systematic review of the differential diagnosis of suspected stroke includes a table listing the 20 most common differential diagnoses. These 20 diagnoses collectively account for nearly 100% of patients with suspected stroke who were not diagnosed with a confirmed transient ischemic attack or stroke. Arranged in the order of frequency, the top 5 non-stroke diagnoses are seizure (19.6%), syncope (12.2%), sepsis (9.6%), benign headache disorder (9%), and brain tumor (8.2%).[58]
Prognosis
The results and conclusions of the following studies highlight the significant impact of acute stroke, irrespective of its type or subtype, and emphasize the importance of collaborative efforts to improve acute stroke care, secondary prevention, and subsequent long-term prognoses.
According to the 2019 systematic analysis of stroke for the Global Burden of Diseases, Injuries, and Risk Factors Study, there was a significant increase in the annual number of strokes (ischemic, ICH, SAH, and all types of strokes combined) and deaths attributed to stroke between 1990 and 2019.[22]
According to a population-based cohort study conducted in the United Kingdom, it was found that 5 years following a first stroke (including ischemic, ICH, SAH, and cases with uncertain type), 47% of patients were deceased, and 39% were living with disabilities.[59]
Based on a longitudinal observational study of the Swedish Stroke Register (Riksstroke), it was found that the survival rates differed between patients with ischemic stroke and those with ICH. At 30 days after a first stroke, 89.9% of patients with ischemic stroke were alive compared to 69.3% of patients with ICH. Although early mortality was higher for patients diagnosed with ICH, beyond 30 days, survival declined at a similar rate for both groups. However, the proportion of functionally dependent survivors remained consistently higher for patients with ICH at all time points. The study found that at 5 years, the survival rate was 49.4% for ischemic stroke and 37.8% for ICH. The authors concluded that 5 years after a first stroke, over 65% of patients with ischemic stroke and over 75% of patients with ICH were either deceased or functionally dependent.[60]
According to a large national cohort study conducted in Australia and New Zealand, among patients hospitalized with a first stroke (including ischemic stroke, ICH, SAH, and cases with unspecified type), the survival rates at 5 years and 10 years were 52.8% and 36.4%, respectively. The study also reported a cumulative incidence of stroke recurrence of 19.8% at 5 years and 26.8% at 10 years.[61]
In a population-based study conducted in the Netherlands, focusing on patients aged 18 to 49 who survived for at least 30 days after experiencing a first stroke (including ischemic stroke, ICH, and stroke not otherwise specified), it was observed that the risk of mortality remained elevated compared to the general population for up to 15 years following the stroke.[62]
In a population-based cohort study conducted in the United Kingdom, it was observed that the rate of stroke recurrence within 5 years decreased from 18% among individuals who experienced a stroke (including ischemic, ICH, and SAH) between 1995 and 1999 to 12% among those who had a stroke between 2000 and 2005. However, there has been no further reduction in stroke recurrence rates since 2005.[63]
A study using Danish Nationwide Health registries revealed that overall recurrence risks following a first ischemic stroke were 4% at 1 year and 13% at 10 years. For the first ICH, the corresponding risks were 3% at 1 year and 12% at 10 years. Moreover, the all-cause mortality risks after a first ischemic stroke were 17% at 1 year and 56% at 10 years, which increased to 25% at 1 year and 70% at 10 years after a recurrent stroke. As for ICH, the all-cause mortality risks were 37% at 1 year and 70% at 10 years after a first stroke, rising to 31% at 1 year and 75% at 10 years after a recurrent stroke.[64]
Complications
Medical complications after stroke significantly cause morbidity and mortality if they are not appropriately anticipated, prevented, and managed.
Ischemic Stroke
Complications related to treating patients with IV alteplase include symptomatic intracranial hemorrhage (6%), major systemic hemorrhage (2%), and angioedema (5%).[11] Clinicians must be prepared to treat these potential emergent adverse effects before drug administration.
Large territorial infarcts can be complicated by brain edema, and depending on the severity, surgical treatment may be required (decompressive surgery has been shown to reduce mortality).[65]
Recurrent seizures after stroke should be treated similarly to when they occur with other acute neurological conditions. Prophylactic use of antiseizure drugs is not recommended.[44]
Intracerebral Hemorrhage
Early identification and prevention of acute medical complications are essential in the first hours and days after ICH. Standardized protocols, including order sets, are recommended to reduce disability and mortality.[39] Additional recommendations include a formal dysphagia screening before initiating oral intake to reduce disability and the risk of pneumonia; hyperpyrexia treatment with antipyretics, standard cooling blankets, water-circulating surface cooling, or catheter-based cooling devices, and; glucose monitoring and prevention of hypoglycemia and hyperglycemia.[39][66][67][68]
Subarachnoid Hemorrhage
Secondary brain injury can occur due to ICH, IVH, ICP, hydrocephalus, subdural hematoma, or DCI.
Medical complications are common and significantly impact outcomes. Reported complications include pyrexia, sepsis, aspiration pneumonia, cardiac dysfunction, anemia, hyponatremia, hyperglycemia, and deep venous thrombosis. Critical care strategies should be directed at prevention, early detection, and targeted treatment, such as maintaining normothermia and normoglycemia.[34][69]
Postoperative and Rehabilitation Care
Rehabilitative therapies are essential within the interdisciplinary team for facilitating a return to activities of daily living and enhancing the overall quality of life during the post-stroke recovery process. However, there is ongoing debate regarding the optimal timing and intensity of rehabilitation services. Some research suggests that very early mobilization (beginning within 24 hours of stroke onset) may harm outcomes and increase the risk of blood pressure-related issues and falls. Nevertheless, further research is required to gain a more comprehensive understanding of this matter.[70][71][72]
Research suggests therapy services should ideally begin within 2 weeks of stroke onset. These services typically include physical, occupational, and speech therapies depending on the specific deficits observed. The primary goals of therapy services include fall prevention, enhancing gait and balance function, contracture and pressure ulcer prevention, return to activities of daily living, and improvement of dysarthria and aphasia, as appropriate to each respective discipline. Mirror therapy, neuromuscular electrical stimulation, task-specific training, proprioceptive neuromuscular facilitation, and cognitive therapeutic exercises are commonly used treatment approaches for addressing stroke-related deficits. Furthermore, transcutaneous electrical stimulation and taping or strapping techniques are used for managing post-stroke shoulder pain. However, further research is necessary to establish optimal rehabilitation approaches across the post-stroke recovery spectrum, as existing research often lacks conclusive evidence or is based on small sample sizes.[70][71][72][73][74]
Deterrence and Patient Education
Acute stroke is both preventable and treatable. Prevention can be accomplished by raising awareness and spreading knowledge of risk factors and warning signs. Public education programs targeting diverse populations, aimed at recognizing the early signs and symptoms of acute stroke and seeking timely emergency care, have proven effective in reducing the time to diagnosis and treatment.[39][75][76]
Modifiable risk factors for stroke include physical inactivity, dyslipidemia, diet and nutrition, hypertension (considered the most important and well-documented), obesity, diabetes mellitus, cigarette smoking, and atrial fibrillation (AF). Therefore, modifications in diet and exercise, smoking cessation, and pharmacotherapy treatment for hypertension, dyslipidemia, diabetes mellitus, and at-risk individuals with AF are essential to acute stroke prevention.[77] Also, to predict stroke risk and guide primary stroke prevention treatment in patients with AF, the CHA2DS2-VASc score is recommended.[77][78]
Stroke is a significant complication associated with sickle cell disease (SCD), particularly in early childhood. Most SCD-related strokes tend to occur in patients with homozygous SCD, making this population a primary target for stroke prevention strategies.[77]
Following an acute stroke, patients should be provided with comprehensive information, counseling, and the opportunity to discuss the impact of stroke on their lives.[44] In addition, caregivers should receive education, support, and training to enhance the quality of life for the patient and themselves.[39]
Enhancing Healthcare Team Outcomes
Interprofessional and multidisciplinary teamwork is necessary to promptly identify and treat patients with acute stroke and provide effective care throughout the recovery and rehabilitation process. Guidelines for ischemic stroke, ICH, and SAH recommend the importance of interprofessional healthcare teams in prehospital and hospital settings to enhance outcomes. Moreover, rehabilitation services provided by a multidisciplinary team during and after hospitalization are essential in facilitating functional recovery in patients and promoting patient independence for individuals who have experienced an acute stroke.[70][79]
The prehospital team usually comprises the Emergency Medical Services and paramedic personnel, with support from the emergency department (ED) clinicians. During the hyperacute phase (first 72 hours post-stroke), essential team members comprise a stroke clinician, a stroke specialist nurse collaborating with ED personnel, and an imaging team led by a radiologist or neuroradiologist.[80] Pharmacists play a crucial role in interprofessional critical care for stroke patients by providing invaluable expertise in recommending appropriate thrombolytic therapy. Their unique position allows them to assess the safety and appropriateness of these medications for individual patients, ensuring accurate dosing and safe preparation. Pharmacists also contribute to medication reconciliation by adequately managing the patient's medications throughout treatment. They also offer vital counseling to patients by guiding them throughout their treatment regimen.
When alerted by a Code: Stroke call, a stroke clinician and specialist nurse commonly attend the ED. Stroke survivors should be directly admitted from the ED to specialized stroke inpatient units to enhance outcomes. This practice reduces mortality rates and promotes increased patient independence during the inpatient stay.[80]
Due to its proven benefits, initiating rehabilitation as early as possible following a stroke is recommended.[70][80] Inpatient teams commonly comprise stroke clinicians, nurses, speech and language therapists, physiotherapists, occupational therapists, and assistants.[80] Early rehabilitation enhances health outcomes, promotes patient confidence, and improves self-care abilities.[79]
Stroke coordinators are valuable interprofessional team members, collaborating with the broader stroke team, stroke survivors, and their families throughout the entire hospitalization period, from admission to discharge. In addition to their role in the team, stroke coordinators may contribute to secondary stroke prevention education by working alongside other team members to provide crucial information and support.[80]
Early supported discharge (ESD) for stroke refers to an interprofessional team intervention designed to enable timely hospital discharge and the provision of stroke-specialized rehabilitation in the patient's home. Internationally, stroke care guidelines recommend ESD services, which initiate treatment within 24 hours of hospital discharge, as an integral component of evidence-based stroke care.[81]
The interprofessional care model, utilizing open communication channels among all care team members, will help achieve optimal patient outcomes for individuals with acute stroke. [Level 5]
Media
References
Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, Elkind MS, George MG, Hamdan AD, Higashida RT, Hoh BL, Janis LS, Kase CS, Kleindorfer DO, Lee JM, Moseley ME, Peterson ED, Turan TN, Valderrama AL, Vinters HV, American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia, Council on Cardiovascular Radiology and Intervention, Council on Cardiovascular and Stroke Nursing, Council on Epidemiology and Prevention, Council on Peripheral Vascular Disease, Council on Nutrition, Physical Activity and Metabolism. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013 Jul:44(7):2064-89. doi: 10.1161/STR.0b013e318296aeca. Epub 2013 May 7 [PubMed PMID: 23652265]
George MG, Fischer L, Koroshetz W, Bushnell C, Frankel M, Foltz J, Thorpe PG. CDC Grand Rounds: Public Health Strategies to Prevent and Treat Strokes. MMWR. Morbidity and mortality weekly report. 2017 May 12:66(18):479-481. doi: 10.15585/mmwr.mm6618a5. Epub 2017 May 12 [PubMed PMID: 28493856]
Katan M, Luft A. Global Burden of Stroke. Seminars in neurology. 2018 Apr:38(2):208-211. doi: 10.1055/s-0038-1649503. Epub 2018 May 23 [PubMed PMID: 29791947]
Ding C, Wu Y, Chen X, Chen Y, Wu Z, Lin Z, Kang D, Fang W, Chen F. Global, regional, and national burden and attributable risk factors of neurological disorders: The Global Burden of Disease study 1990-2019. Frontiers in public health. 2022:10():952161. doi: 10.3389/fpubh.2022.952161. Epub 2022 Nov 29 [PubMed PMID: 36523572]
Struijs JN, van Genugten ML, Evers SM, Ament AJ, Baan CA, van den Bos GA. Future costs of stroke in the Netherlands: the impact of stroke services. International journal of technology assessment in health care. 2006 Fall:22(4):518-24 [PubMed PMID: 16984687]
Luengo-Fernandez R, Violato M, Candio P, Leal J. Economic burden of stroke across Europe: A population-based cost analysis. European stroke journal. 2020 Mar:5(1):17-25. doi: 10.1177/2396987319883160. Epub 2019 Oct 29 [PubMed PMID: 32232166]
Rochmah TN, Rahmawati IT, Dahlui M, Budiarto W, Bilqis N. Economic Burden of Stroke Disease: A Systematic Review. International journal of environmental research and public health. 2021 Jul 15:18(14):. doi: 10.3390/ijerph18147552. Epub 2021 Jul 15 [PubMed PMID: 34299999]
Level 2 (mid-level) evidenceHankey GJ. Stroke. Lancet (London, England). 2017 Feb 11:389(10069):641-654. doi: 10.1016/S0140-6736(16)30962-X. Epub 2016 Sep 13 [PubMed PMID: 27637676]
Radu RA, Terecoasă EO, Băjenaru OA, Tiu C. Etiologic classification of ischemic stroke: Where do we stand? Clinical neurology and neurosurgery. 2017 Aug:159():93-106. doi: 10.1016/j.clineuro.2017.05.019. Epub 2017 May 24 [PubMed PMID: 28609703]
Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993 Jan:24(1):35-41 [PubMed PMID: 7678184]
Level 1 (high-level) evidenceChugh C. Acute Ischemic Stroke: Management Approach. Indian journal of critical care medicine : peer-reviewed, official publication of Indian Society of Critical Care Medicine. 2019 Jun:23(Suppl 2):S140-S146. doi: 10.5005/jp-journals-10071-23192. Epub [PubMed PMID: 31485123]
Feske SK. Ischemic Stroke. The American journal of medicine. 2021 Dec:134(12):1457-1464. doi: 10.1016/j.amjmed.2021.07.027. Epub 2021 Aug 27 [PubMed PMID: 34454905]
Yamamoto Y, Ohara T, Hamanaka M, Hosomi A, Tamura A, Akiguchi I. Characteristics of intracranial branch atheromatous disease and its association with progressive motor deficits. Journal of the neurological sciences. 2011 May 15:304(1-2):78-82. doi: 10.1016/j.jns.2011.02.006. Epub 2011 Mar 13 [PubMed PMID: 21402390]
Yaghi S, Raz E, Yang D, Cutting S, Mac Grory B, Elkind MS, de Havenon A. Lacunar stroke: mechanisms and therapeutic implications. Journal of neurology, neurosurgery, and psychiatry. 2021 May 26:():. pii: jnnp-2021-326308. doi: 10.1136/jnnp-2021-326308. Epub 2021 May 26 [PubMed PMID: 34039632]
Kremer PH, Jolink WM, Kappelle LJ, Algra A, Klijn CJ, SMART and ESPRIT Study Groups. Risk Factors for Lobar and Non-Lobar Intracerebral Hemorrhage in Patients with Vascular Disease. PloS one. 2015:10(11):e0142338. doi: 10.1371/journal.pone.0142338. Epub 2015 Nov 5 [PubMed PMID: 26540190]
An SJ, Kim TJ, Yoon BW. Epidemiology, Risk Factors, and Clinical Features of Intracerebral Hemorrhage: An Update. Journal of stroke. 2017 Jan:19(1):3-10. doi: 10.5853/jos.2016.00864. Epub 2017 Jan 31 [PubMed PMID: 28178408]
Magid-Bernstein J, Girard R, Polster S, Srinath A, Romanos S, Awad IA, Sansing LH. Cerebral Hemorrhage: Pathophysiology, Treatment, and Future Directions. Circulation research. 2022 Apr 15:130(8):1204-1229. doi: 10.1161/CIRCRESAHA.121.319949. Epub 2022 Apr 14 [PubMed PMID: 35420918]
Level 3 (low-level) evidenceMartin CO, Rymer MM. Hemorrhagic stroke: aneurysmal subarachnoid hemorrhage. Missouri medicine. 2011 Mar-Apr:108(2):124-7 [PubMed PMID: 21568235]
Sweeney K, Silver N, Javadpour M. Subarachnoid haemorrhage (spontaneous aneurysmal). BMJ clinical evidence. 2016 Mar 17:2016():. pii: 1213. Epub 2016 Mar 17 [PubMed PMID: 26983641]
Maher M, Schweizer TA, Macdonald RL. Treatment of Spontaneous Subarachnoid Hemorrhage: Guidelines and Gaps. Stroke. 2020 Apr:51(4):1326-1332. doi: 10.1161/STROKEAHA.119.025997. Epub 2020 Jan 22 [PubMed PMID: 31964292]
Treadwell SD, Robinson TG. Cocaine use and stroke. Postgraduate medical journal. 2007 Jun:83(980):389-94 [PubMed PMID: 17551070]
Level 3 (low-level) evidenceGBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet. Neurology. 2021 Oct:20(10):795-820. doi: 10.1016/S1474-4422(21)00252-0. Epub 2021 Sep 3 [PubMed PMID: 34487721]
Level 1 (high-level) evidenceGBD 2016 Lifetime Risk of Stroke Collaborators, Feigin VL, Nguyen G, Cercy K, Johnson CO, Alam T, Parmar PG, Abajobir AA, Abate KH, Abd-Allah F, Abejie AN, Abyu GY, Ademi Z, Agarwal G, Ahmed MB, Akinyemi RO, Al-Raddadi R, Aminde LN, Amlie-Lefond C, Ansari H, Asayesh H, Asgedom SW, Atey TM, Ayele HT, Banach M, Banerjee A, Barac A, Barker-Collo SL, Bärnighausen T, Barregard L, Basu S, Bedi N, Behzadifar M, Béjot Y, Bennett DA, Bensenor IM, Berhe DF, Boneya DJ, Brainin M, Campos-Nonato IR, Caso V, Castañeda-Orjuela CA, Rivas JC, Catalá-López F, Christensen H, Criqui MH, Damasceno A, Dandona L, Dandona R, Davletov K, de Courten B, deVeber G, Dokova K, Edessa D, Endres M, Faraon EJA, Farvid MS, Fischer F, Foreman K, Forouzanfar MH, Gall SL, Gebrehiwot TT, Geleijnse JM, Gillum RF, Giroud M, Goulart AC, Gupta R, Gupta R, Hachinski V, Hamadeh RR, Hankey GJ, Hareri HA, Havmoeller R, Hay SI, Hegazy MI, Hibstu DT, James SL, Jeemon P, John D, Jonas JB, Jóźwiak J, Kalani R, Kandel A, Kasaeian A, Kengne AP, Khader YS, Khan AR, Khang YH, Khubchandani J, Kim D, Kim YJ, Kivimaki M, Kokubo Y, Kolte D, Kopec JA, Kosen S, Kravchenko M, Krishnamurthi R, Kumar GA, Lafranconi A, Lavados PM, Legesse Y, Li Y, Liang X, Lo WD, Lorkowski S, Lotufo PA, Loy CT, Mackay MT, Abd El Razek HM, Mahdavi M, Majeed A, Malekzadeh R, Malta DC, Mamun AA, Mantovani LG, Martins SCO, Mate KK, Mazidi M, Mehata S, Meier T, Melaku YA, Mendoza W, Mensah GA, Meretoja A, Mezgebe HB, Miazgowski T, Miller TR, Ibrahim NM, Mohammed S, Mokdad AH, Moosazadeh M, Moran AE, Musa KI, Negoi RI, Nguyen M, Nguyen QL, Nguyen TH, Tran TT, Nguyen TT, Anggraini Ningrum DN, Norrving B, Noubiap JJ, O’Donnell MJ, Olagunju AT, Onuma OK, Owolabi MO, Parsaeian M, Patton GC, Piradov M, Pletcher MA, Pourmalek F, Prakash V, Qorbani M, Rahman M, Rahman MA, Rai RK, Ranta A, Rawaf D, Rawaf S, Renzaho AM, Robinson SR, Sahathevan R, Sahebkar A, Salomon JA, Santalucia P, Santos IS, Sartorius B, Schutte AE, Sepanlou SG, Shafieesabet A, Shaikh MA, Shamsizadeh M, Sheth KN, Sisay M, Shin MJ, Shiue I, Silva DAS, Sobngwi E, Soljak M, Sorensen RJD, Sposato LA, Stranges S, Suliankatchi RA, Tabarés-Seisdedos R, Tanne D, Nguyen CT, Thakur JS, Thrift AG, Tirschwell DL, Topor-Madry R, Tran BX, Nguyen LT, Truelsen T, Tsilimparis N, Tyrovolas S, Ukwaja KN, Uthman OA, Varakin Y, Vasankari T, Venketasubramanian N, Vlassov VV, Wang W, Werdecker A, Wolfe CDA, Xu G, Yano Y, Yonemoto N, Yu C, Zaidi Z, El Sayed Zaki M, Zhou M, Ziaeian B, Zipkin B, Vos T, Naghavi M, Murray CJL, Roth GA. Global, Regional, and Country-Specific Lifetime Risks of Stroke, 1990 and 2016. The New England journal of medicine. 2018 Dec 20:379(25):2429-2437. doi: 10.1056/NEJMoa1804492. Epub [PubMed PMID: 30575491]
Saver JL. Penumbral salvage and thrombolysis outcome: a drop of brain, a week of life. Brain : a journal of neurology. 2017 Mar 1:140(3):519-522. doi: 10.1093/brain/awx020. Epub [PubMed PMID: 28364555]
Xing C, Arai K, Lo EH, Hommel M. Pathophysiologic cascades in ischemic stroke. International journal of stroke : official journal of the International Stroke Society. 2012 Jul:7(5):378-85. doi: 10.1111/j.1747-4949.2012.00839.x. Epub [PubMed PMID: 22712739]
Lalancette-Hébert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007 Mar 7:27(10):2596-605 [PubMed PMID: 17344397]
Level 3 (low-level) evidenceDenes A, Vidyasagar R, Feng J, Narvainen J, McColl BW, Kauppinen RA, Allan SM. Proliferating resident microglia after focal cerebral ischaemia in mice. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2007 Dec:27(12):1941-53 [PubMed PMID: 17440490]
Level 3 (low-level) evidenceBattista D, Ferrari CC, Gage FH, Pitossi FJ. Neurogenic niche modulation by activated microglia: transforming growth factor beta increases neurogenesis in the adult dentate gyrus. The European journal of neuroscience. 2006 Jan:23(1):83-93 [PubMed PMID: 16420418]
Level 3 (low-level) evidenceNarantuya D, Nagai A, Sheikh AM, Masuda J, Kobayashi S, Yamaguchi S, Kim SU. Human microglia transplanted in rat focal ischemia brain induce neuroprotection and behavioral improvement. PloS one. 2010 Jul 23:5(7):e11746. doi: 10.1371/journal.pone.0011746. Epub 2010 Jul 23 [PubMed PMID: 20668522]
Level 3 (low-level) evidenceMerson TD, Binder MD, Kilpatrick TJ. Role of cytokines as mediators and regulators of microglial activity in inflammatory demyelination of the CNS. Neuromolecular medicine. 2010 Jun:12(2):99-132. doi: 10.1007/s12017-010-8112-z. Epub 2010 Mar 30 [PubMed PMID: 20411441]
Level 3 (low-level) evidenceKiefer R, Streit WJ, Toyka KV, Kreutzberg GW, Hartung HP. Transforming growth factor-beta 1: a lesion-associated cytokine of the nervous system. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 1995 Jun-Jul:13(3-4):331-9 [PubMed PMID: 7572285]
Level 3 (low-level) evidenceAronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke. 2011 Jun:42(6):1781-6. doi: 10.1161/STROKEAHA.110.596718. Epub 2011 Apr 28 [PubMed PMID: 21527759]
Wilkinson DA, Pandey AS, Thompson BG, Keep RF, Hua Y, Xi G. Injury mechanisms in acute intracerebral hemorrhage. Neuropharmacology. 2018 May 15:134(Pt B):240-248. doi: 10.1016/j.neuropharm.2017.09.033. Epub 2017 Sep 22 [PubMed PMID: 28947377]
Claassen J, Park S. Spontaneous subarachnoid haemorrhage. Lancet (London, England). 2022 Sep 10:400(10355):846-862. doi: 10.1016/S0140-6736(22)00938-2. Epub 2022 Aug 16 [PubMed PMID: 35985353]
H Buck B, Akhtar N, Alrohimi A, Khan K, Shuaib A. Stroke mimics: incidence, aetiology, clinical features and treatment. Annals of medicine. 2021 Dec:53(1):420-436. doi: 10.1080/07853890.2021.1890205. Epub [PubMed PMID: 33678099]
Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet (London, England). 2009 May 9:373(9675):1632-44. doi: 10.1016/S0140-6736(09)60371-8. Epub [PubMed PMID: 19427958]
Vespa PM, O'Phelan K, Shah M, Mirabelli J, Starkman S, Kidwell C, Saver J, Nuwer MR, Frazee JG, McArthur DA, Martin NA. Acute seizures after intracerebral hemorrhage: a factor in progressive midline shift and outcome. Neurology. 2003 May 13:60(9):1441-6 [PubMed PMID: 12743228]
Level 2 (mid-level) evidenceDe Herdt V, Dumont F, Hénon H, Derambure P, Vonck K, Leys D, Cordonnier C. Early seizures in intracerebral hemorrhage: incidence, associated factors, and outcome. Neurology. 2011 Nov 15:77(20):1794-800. doi: 10.1212/WNL.0b013e31823648a6. Epub 2011 Oct 5 [PubMed PMID: 21975203]
Greenberg SM, Ziai WC, Cordonnier C, Dowlatshahi D, Francis B, Goldstein JN, Hemphill JC 3rd, Johnson R, Keigher KM, Mack WJ, Mocco J, Newton EJ, Ruff IM, Sansing LH, Schulman S, Selim MH, Sheth KN, Sprigg N, Sunnerhagen KS, American Heart Association/American Stroke Association. 2022 Guideline for the Management of Patients With Spontaneous Intracerebral Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke. 2022 Jul:53(7):e282-e361. doi: 10.1161/STR.0000000000000407. Epub 2022 May 17 [PubMed PMID: 35579034]
MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, Abbott R, Godwin J, Dyer A, Stamler J. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet (London, England). 1990 Mar 31:335(8692):765-74 [PubMed PMID: 1969518]
Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet (London, England). 2002 Dec 14:360(9349):1903-13 [PubMed PMID: 12493255]
Level 1 (high-level) evidenceSuarez JI. Diagnosis and Management of Subarachnoid Hemorrhage. Continuum (Minneapolis, Minn.). 2015 Oct:21(5 Neurocritical Care):1263-87. doi: 10.1212/CON.0000000000000217. Epub [PubMed PMID: 26426230]
Lövblad KO, Altrichter S, Mendes Pereira V, Vargas M, Marcos Gonzalez A, Haller S, Sztajzel R. Imaging of acute stroke: CT and/or MRI. Journal of neuroradiology = Journal de neuroradiologie. 2015 Feb:42(1):55-64. doi: 10.1016/j.neurad.2014.10.005. Epub 2014 Nov 25 [PubMed PMID: 25466468]
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019 Dec:50(12):e344-e418. doi: 10.1161/STR.0000000000000211. Epub 2019 Oct 30 [PubMed PMID: 31662037]
Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA, Sila CA, Hassan AE, Millan M, Levy EI, Mitchell P, Chen M, English JD, Shah QA, Silver FL, Pereira VM, Mehta BP, Baxter BW, Abraham MG, Cardona P, Veznedaroglu E, Hellinger FR, Feng L, Kirmani JF, Lopes DK, Jankowitz BT, Frankel MR, Costalat V, Vora NA, Yoo AJ, Malik AM, Furlan AJ, Rubiera M, Aghaebrahim A, Olivot JM, Tekle WG, Shields R, Graves T, Lewis RJ, Smith WS, Liebeskind DS, Saver JL, Jovin TG, DAWN Trial Investigators. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. The New England journal of medicine. 2018 Jan 4:378(1):11-21. doi: 10.1056/NEJMoa1706442. Epub 2017 Nov 11 [PubMed PMID: 29129157]
Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, McTaggart RA, Torbey MT, Kim-Tenser M, Leslie-Mazwi T, Sarraj A, Kasner SE, Ansari SA, Yeatts SD, Hamilton S, Mlynash M, Heit JJ, Zaharchuk G, Kim S, Carrozzella J, Palesch YY, Demchuk AM, Bammer R, Lavori PW, Broderick JP, Lansberg MG, DEFUSE 3 Investigators. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. The New England journal of medicine. 2018 Feb 22:378(8):708-718. doi: 10.1056/NEJMoa1713973. Epub 2018 Jan 24 [PubMed PMID: 29364767]
Thomalla G, Simonsen CZ, Boutitie F, Andersen G, Berthezene Y, Cheng B, Cheripelli B, Cho TH, Fazekas F, Fiehler J, Ford I, Galinovic I, Gellissen S, Golsari A, Gregori J, Günther M, Guibernau J, Häusler KG, Hennerici M, Kemmling A, Marstrand J, Modrau B, Neeb L, Perez de la Ossa N, Puig J, Ringleb P, Roy P, Scheel E, Schonewille W, Serena J, Sunaert S, Villringer K, Wouters A, Thijs V, Ebinger M, Endres M, Fiebach JB, Lemmens R, Muir KW, Nighoghossian N, Pedraza S, Gerloff C, WAKE-UP Investigators. MRI-Guided Thrombolysis for Stroke with Unknown Time of Onset. The New England journal of medicine. 2018 Aug 16:379(7):611-622. doi: 10.1056/NEJMoa1804355. Epub 2018 May 16 [PubMed PMID: 29766770]
Perry JJ, Sivilotti MLA, Émond M, Hohl CM, Khan M, Lesiuk H, Abdulaziz K, Wells GA, Stiell IG. Prospective Implementation of the Ottawa Subarachnoid Hemorrhage Rule and 6-Hour Computed Tomography Rule. Stroke. 2020 Feb:51(2):424-430. doi: 10.1161/STROKEAHA.119.026969. Epub 2019 Dec 6 [PubMed PMID: 31805846]
Cho WS, Kim JE, Park SQ, Ko JK, Kim DW, Park JC, Yeon JY, Chung SY, Chung J, Joo SP, Hwang G, Kim DY, Chang WH, Choi KS, Lee SH, Sheen SH, Kang HS, Kim BM, Bae HJ, Oh CW, Park HS, Quality Control Committees from the Korean Society of Cerebrovascular Surgeons, Society of Korean Endovascular Neurosurgeons, Korean Society of Interventional Neuroradiology, Korean Stroke Society and Korean Academy of Rehabilitation Medicine. Korean Clinical Practice Guidelines for Aneurysmal Subarachnoid Hemorrhage. Journal of Korean Neurosurgical Society. 2018 Mar:61(2):127-166. doi: 10.3340/jkns.2017.0404.005. Epub 2018 Feb 28 [PubMed PMID: 29526058]
Level 2 (mid-level) evidenceConnolly ES Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, Hoh BL, Kirkness CJ, Naidech AM, Ogilvy CS, Patel AB, Thompson BG, Vespa P, American Heart Association Stroke Council, Council on Cardiovascular Radiology and Intervention, Council on Cardiovascular Nursing, Council on Cardiovascular Surgery and Anesthesia, Council on Clinical Cardiology. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012 Jun:43(6):1711-37. doi: 10.1161/STR.0b013e3182587839. Epub 2012 May 3 [PubMed PMID: 22556195]
Thomalla G, Gerloff C. Acute imaging for evidence-based treatment of ischemic stroke. Current opinion in neurology. 2019 Aug:32(4):521-529. doi: 10.1097/WCO.0000000000000716. Epub [PubMed PMID: 31116116]
Level 3 (low-level) evidenceHanger HC, Geddes JA, Wilkinson TJ, Lee M, Baker AE. Warfarin-related intracerebral haemorrhage: better outcomes when reversal includes prothrombin complex concentrates. Internal medicine journal. 2013 Mar:43(3):308-16. doi: 10.1111/imj.12034. Epub [PubMed PMID: 23176226]
Level 3 (low-level) evidenceLi X, Sun Z, Zhao W, Zhang J, Chen J, Li Y, Ye Y, Zhao J, Yang X, Xiang Y, Li G, Mao J, Zhang W, Zhang M, Zhang W. Effect of acetylsalicylic acid usage and platelet transfusion on postoperative hemorrhage and activities of daily living in patients with acute intracerebral hemorrhage. Journal of neurosurgery. 2013 Jan:118(1):94-103. doi: 10.3171/2012.9.JNS112286. Epub 2012 Oct 19 [PubMed PMID: 23082885]
Level 1 (high-level) evidenceBaharoglu MI, Cordonnier C, Al-Shahi Salman R, de Gans K, Koopman MM, Brand A, Majoie CB, Beenen LF, Marquering HA, Vermeulen M, Nederkoorn PJ, de Haan RJ, Roos YB, PATCH Investigators. Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): a randomised, open-label, phase 3 trial. Lancet (London, England). 2016 Jun 25:387(10038):2605-2613. doi: 10.1016/S0140-6736(16)30392-0. Epub 2016 May 10 [PubMed PMID: 27178479]
Level 1 (high-level) evidenceSteiner T, Juvela S, Unterberg A, Jung C, Forsting M, Rinkel G, European Stroke Organization. European Stroke Organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovascular diseases (Basel, Switzerland). 2013:35(2):93-112. doi: 10.1159/000346087. Epub 2013 Feb 7 [PubMed PMID: 23406828]
Rawal S, Alcaide-Leon P, Macdonald RL, Rinkel GJ, Victor JC, Krings T, Kapral MK, Laupacis A. Meta-analysis of timing of endovascular aneurysm treatment in subarachnoid haemorrhage: inconsistent results of early treatment within 1 day. Journal of neurology, neurosurgery, and psychiatry. 2017 Mar:88(3):241-248. doi: 10.1136/jnnp-2016-314596. Epub 2017 Jan 18 [PubMed PMID: 28100721]
Level 1 (high-level) evidenceShi M, Yang C, Chen ZH, Xiao LF, Zhao WY. Efficacy and Safety of Tranexamic Acid in Aneurysmal Subarachnoid Hemorrhage: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Frontiers in surgery. 2021:8():790149. doi: 10.3389/fsurg.2021.790149. Epub 2022 Jan 10 [PubMed PMID: 35083272]
Level 1 (high-level) evidenceGibson LM, Whiteley W. The differential diagnosis of suspected stroke: a systematic review. The journal of the Royal College of Physicians of Edinburgh. 2013:43(2):114-8. doi: 10.4997/JRCPE.2013.205. Epub [PubMed PMID: 23734351]
Level 1 (high-level) evidenceLuengo-Fernandez R, Paul NL, Gray AM, Pendlebury ST, Bull LM, Welch SJ, Cuthbertson FC, Rothwell PM, Oxford Vascular Study. Population-based study of disability and institutionalization after transient ischemic attack and stroke: 10-year results of the Oxford Vascular Study. Stroke. 2013 Oct:44(10):2854-61. doi: 10.1161/STROKEAHA.113.001584. Epub 2013 Aug 6 [PubMed PMID: 23920019]
Sennfält S, Norrving B, Petersson J, Ullberg T. Long-Term Survival and Function After Stroke: A Longitudinal Observational Study From the Swedish Stroke Register. Stroke. 2019 Jan:50(1):53-61. doi: 10.1161/STROKEAHA.118.022913. Epub 2018 Dec 7 [PubMed PMID: 30580719]
Peng Y, Ngo L, Hay K, Alghamry A, Colebourne K, Ranasinghe I. Long-Term Survival, Stroke Recurrence, and Life Expectancy After an Acute Stroke in Australia and New Zealand From 2008-2017: A Population-Wide Cohort Study. Stroke. 2022 Aug:53(8):2538-2548. doi: 10.1161/STROKEAHA.121.038155. Epub 2022 Apr 14 [PubMed PMID: 35418238]
Ekker MS, Verhoeven JI, Vaartjes I, Jolink WMT, Klijn CJM, de Leeuw FE. Association of Stroke Among Adults Aged 18 to 49 Years With Long-term Mortality. JAMA. 2019 Jun 4:321(21):2113-2123. doi: 10.1001/jama.2019.6560. Epub [PubMed PMID: 31121602]
Flach C, Muruet W, Wolfe CDA, Bhalla A, Douiri A. Risk and Secondary Prevention of Stroke Recurrence: A Population-Base Cohort Study. Stroke. 2020 Aug:51(8):2435-2444. doi: 10.1161/STROKEAHA.120.028992. Epub 2020 Jul 10 [PubMed PMID: 32646337]
Skajaa N, Adelborg K, Horváth-Puhó E, Rothman KJ, Henderson VW, Thygesen LC, Sørensen HT. Risks of Stroke Recurrence and Mortality After First and Recurrent Strokes in Denmark: A Nationwide Registry Study. Neurology. 2021 Nov 29:():. pii: 10.1212/WNL.0000000000013118. doi: 10.1212/WNL.0000000000013118. Epub 2021 Nov 29 [PubMed PMID: 34845054]
Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A, Amelink GJ, Schmiedeck P, Schwab S, Rothwell PM, Bousser MG, van der Worp HB, Hacke W, DECIMAL, DESTINY, and HAMLET investigators. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. The Lancet. Neurology. 2007 Mar:6(3):215-22 [PubMed PMID: 17303527]
Level 1 (high-level) evidenceRincon F, Mayer SA. Clinical review: Critical care management of spontaneous intracerebral hemorrhage. Critical care (London, England). 2008:12(6):237. doi: 10.1186/cc7092. Epub 2008 Dec 10 [PubMed PMID: 19108704]
Diringer MN, Neurocritical Care Fever Reduction Trial Group. Treatment of fever in the neurologic intensive care unit with a catheter-based heat exchange system. Critical care medicine. 2004 Feb:32(2):559-64 [PubMed PMID: 14758179]
Level 1 (high-level) evidenceMayer SA, Kowalski RG, Presciutti M, Ostapkovich ND, McGann E, Fitzsimmons BF, Yavagal DR, Du YE, Naidech AM, Janjua NA, Claassen J, Kreiter KT, Parra A, Commichau C. Clinical trial of a novel surface cooling system for fever control in neurocritical care patients. Critical care medicine. 2004 Dec:32(12):2508-15 [PubMed PMID: 15599159]
Level 1 (high-level) evidenceHammer A, Ranaie G, Erbguth F, Hohenhaus M, Wenzl M, Killer-Oberpfalzer M, Steiner HH, Janssen H. Impact of Complications and Comorbidities on the Intensive Care Length of Stay after Aneurysmal Subarachnoid Haemorrhage. Scientific reports. 2020 Apr 10:10(1):6228. doi: 10.1038/s41598-020-63298-9. Epub 2020 Apr 10 [PubMed PMID: 32277142]
Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, Deruyter F, Eng JJ, Fisher B, Harvey RL, Lang CE, MacKay-Lyons M, Ottenbacher KJ, Pugh S, Reeves MJ, Richards LG, Stiers W, Zorowitz RD, American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2016 Jun:47(6):e98-e169. doi: 10.1161/STR.0000000000000098. Epub 2016 May 4 [PubMed PMID: 27145936]
Level 2 (mid-level) evidenceColeman ER, Moudgal R, Lang K, Hyacinth HI, Awosika OO, Kissela BM, Feng W. Early Rehabilitation After Stroke: a Narrative Review. Current atherosclerosis reports. 2017 Nov 7:19(12):59. doi: 10.1007/s11883-017-0686-6. Epub 2017 Nov 7 [PubMed PMID: 29116473]
Level 3 (low-level) evidenceNational Collaborating Centre for Chronic Conditions (UK). Stroke: National Clinical Guideline for Diagnosis and Initial Management of Acute Stroke and Transient Ischaemic Attack (TIA). 2008:(): [PubMed PMID: 21698846]
Almhdawi KA, Mathiowetz VG, White M, delMas RC. Efficacy of Occupational Therapy Task-oriented Approach in Upper Extremity Post-stroke Rehabilitation. Occupational therapy international. 2016 Dec:23(4):444-456. doi: 10.1002/oti.1447. Epub 2016 Oct 20 [PubMed PMID: 27761966]
Gandhi DB, Sterba A, Khatter H, Pandian JD. Mirror Therapy in Stroke Rehabilitation: Current Perspectives. Therapeutics and clinical risk management. 2020:16():75-85. doi: 10.2147/TCRM.S206883. Epub 2020 Feb 7 [PubMed PMID: 32103968]
Level 3 (low-level) evidencePatel A, Fang J, Gillespie C, Odom E, King SC, Luncheon C, Ayala C. Awareness of Stroke Signs and Symptoms and Calling 9-1-1 Among US Adults: National Health Interview Survey, 2009 and 2014. Preventing chronic disease. 2019 Jun 20:16():E78. doi: 10.5888/pcd16.180564. Epub 2019 Jun 20 [PubMed PMID: 31228234]
Level 3 (low-level) evidenceSirisha S, Jala S, Vooturi S, Yada PK, Kaul S. Awareness, Recognition, and Response to Stroke among the General Public-An Observational Study. Journal of neurosciences in rural practice. 2021 Oct:12(4):704-710. doi: 10.1055/s-0041-1735822. Epub 2021 Sep 23 [PubMed PMID: 34737504]
Level 2 (mid-level) evidenceMeschia JF, Bushnell C, Boden-Albala B, Braun LT, Bravata DM, Chaturvedi S, Creager MA, Eckel RH, Elkind MS, Fornage M, Goldstein LB, Greenberg SM, Horvath SE, Iadecola C, Jauch EC, Moore WS, Wilson JA, American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, Council on Functional Genomics and Translational Biology, Council on Hypertension. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014 Dec:45(12):3754-832. doi: 10.1161/STR.0000000000000046. Epub 2014 Oct 28 [PubMed PMID: 25355838]
Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GY, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2016 Nov:18(11):1609-1678 [PubMed PMID: 27567465]
Chiu CC, Lin HF, Lin CH, Chang HT, Hsien HH, Hung KW, Tung SL, Shi HY. Multidisciplinary Care after Acute Care for Stroke: A Prospective Comparison between a Multidisciplinary Post-Acute Care Group and a Standard Group Matched by Propensity Score. International journal of environmental research and public health. 2021 Jul 20:18(14):. doi: 10.3390/ijerph18147696. Epub 2021 Jul 20 [PubMed PMID: 34300144]
Clarke DJ, Forster A. Improving post-stroke recovery: the role of the multidisciplinary health care team. Journal of multidisciplinary healthcare. 2015:8():433-42. doi: 10.2147/JMDH.S68764. Epub 2015 Sep 22 [PubMed PMID: 26445548]
Fisher RJ, Byrne A, Chouliara N, Lewis S, Paley L, Hoffman A, Rudd A, Robinson T, Langhorne P, Walker MF. Effectiveness of Stroke Early Supported Discharge: Analysis From a National Stroke Registry. Circulation. Cardiovascular quality and outcomes. 2020 Aug:13(8):e006395. doi: 10.1161/CIRCOUTCOMES.119.006395. Epub 2020 Jul 17 [PubMed PMID: 32674640]
Level 2 (mid-level) evidence