Indications
Hypertrophic cardiomyopathy (HCM) is the most common heritable cardiac disorder.[1] This monogenetic disease is characterized by left ventricular hypertrophy in the absence of secondary causes, such as increased loading conditions (eg, hypertension or valvular pathology) or infiltrative disorders. HCM is an autosomal dominant disease associated with cardiac sarcomeres, myosin-binding protein C, and β-myosin heavy chain mutations.[2]
Initially asymptomatic, HCM is characterized by a gradual progression of shortness of breath on exertion and worsening quality of life due to dynamic obstruction of the left ventricular outflow tract (LVOT). Obstructive HCM arises from myofibrillar disarray, leading to septal hypertrophy and an abnormal subvalvular mitral apparatus, which causes systolic anterior motion of one or both mitral leaflets.[1] The prognosis for HCM is variable, with symptomatic heart failure and sudden cardiac death occurring in a small subset of patients.[1]
FDA-Approved Indications
Mavacamten is indicated for adults with symptomatic heart failure classified as New York Heart Association (NYHA) class II and III secondary to obstructive HCM to improve functional capacity and symptoms. The drug received approval from the US Food and Drug Administration (FDA) in 2022 following the publication of the phase III pivotal clinical study EXPLORER-HCM (Evaluate Mavacamten in Adults with Symptomatic Obstructive Hypertrophic Cardiomyopathy).[3]
In the EXPLORER trial, obstructive HCM was defined as unexplained left ventricular hypertrophy with a left ventricular wall thickness of 15 mm or more and a peak LVOT gradient of at least 50 mm Hg at rest. All patients had a left ventricular ejection fraction (LVEF) of at least 55% and were aged 18 or older.[3] Patients on dual therapy with a β-blocker and calcium channel blocker, as well as those on monotherapy with disopyramide or ranolazine, were excluded. Patients with known infiltrative or storage diseases causing left ventricular hypertrophy were not included. After 62 weeks of therapy, mavacamten was associated with a decreased resting and post-exercise LVOT gradient, improved NYHA heart failure symptom classification, and enhanced exercise performance.
In addition, a post-exercise LVOT gradient of less than 30 mm Hg was achieved in 57% of patients in the mavacamten group, representing a 50% greater reduction compared to the placebo group. The mavacamten group also demonstrated a 34% greater reduction in functional class compared to the placebo group. Furthermore, mavacamten use in patients with obstructive HCM was associated with significant improvements in quality of life, as measured by various health-related quality of life indexes, including the Kansas City Cardiomyopathy Questionnaire Overall Summary Score and the Hypertrophic Cardiomyopathy Symptom Questionnaire Shortness of Breath.[4]
Cardiac magnetic resonance imaging analysis has shown that mavacamten is associated with absolute reductions in the intracellular myocardial mass index, with no changes in myocardial contractile fraction or fibrosis after 30 weeks of therapy.[5] Statistical projections using a Markov model have evaluated the long-term net health benefits of mavacamten in patients with obstructive HCM. These findings suggest that mavacamten treatment is associated with an incremental increase in both years of life and years spent in NYHA functional class I, compared to current treatment modalities for obstructive HCM.[6]
The use of mavacamten has also been proposed for patients with nonobstructive HCM. The MAVERICK-HCM trial (Mavacamten in Adults with Symptomatic Nonobstructive Hypertrophic Cardiomyopathy) explored the safety and efficacy of mavacamten in this subset of patients. In this phase II study, mavacamten treatment was associated with a significant reduction in myocardial wall stress markers, such as cardiac troponin I and N-terminal pro-B-type natriuretic peptide.[7] However, the analysis's short treatment period and dose-finding nature precluded the assessment of clinically significant differences in symptoms or quality of life compared to placebo.
In patients with obstructive HCM and NYHA class IV symptoms who are eligible for septal reduction therapies (SRTs), mavacamten was evaluated in the VALOR-HCM trial (Evaluation of Mavacamten in Adults With Symptomatic Obstructive Hypertrophic Cardiomyopathy who are Eligible for Septal Reduction Therapy).[8] The trial demonstrated that mavacamten effectively reduced the need for SRT by week 56. This outcome was supported by sustained improvements in LVOT gradients and symptomatic relief, highlighting mavacamten's potential as a valuable treatment option.[9]
A systematic review demonstrated that mavacamten improves symptoms of HCM, enhances peak oxygen consumption (pVO2), and decreases post-exercise LVOT gradient. Despite some observed adverse effects, such as atrial fibrillation and transient decreases in LVEF, mavacamten is generally well-tolerated.[10] According to the American College of Cardiology (ACC) 2024 guidelines, mavacamten should be used in patients with symptomatic obstructive HCM who do not achieve sufficient relief from first-line pharmacotherapy, such as β-blockers or non-dihydropyridine calcium channel blockers.[11]
Off-Label Uses
Mavacamten has also been proposed for patients with nonobstructive HCM. The MAVERICK-HCM trial investigated the safety and efficacy of mavacamten in this patient population. The treatment was associated with a significant reduction in myocardial wall stress markers, such as cardiac troponin I and N-terminal pro-B-type natriuretic peptide.[7] However, the short treatment period and dose-finding nature of this analysis precluded the assessment of clinically significant differences in symptoms or quality of life compared to a placebo.
Mechanism of Action
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Mechanism of Action
Mavacamten is an allosteric, selective, and reversible inhibitor of cardiac myosin ATPase.[3][12] This first-in-class medication reduces the formation of actin-myosin cross-bridges, thus reducing the probability of systolic and diastolic cross-bridge formation. Excessive myosin-actin cross-bridge arrangement and dysregulation of the relaxed state are characteristic of HCM. Mavacamten promotes an energy-sparing and super-relaxed state that translates as a reduction in LVOT obstruction and improvement of cardiac filling pressures.[13]
Pharmacokinetics
Absorption: Mavacamten is rapidly absorbed with an oral bioavailability of 85%. Meal timing does not affect its absorption, and the time to maximum concentration (Tmax) is 1 to 2 hours.
Distribution: The plasma protein binding of mavacamten is approximately 97%.[14]
Metabolism: Mavacamten is primarily metabolized by CYP2C19 and, to a lesser extent, by CYP3A4. The accumulation of mavacamten is significantly influenced by CYP2C19 metabolism status, particularly, with the most pronounced accumulation occurring in patients who are poor metabolizers of CYP2C19.[15][16]
Elimination: More than 80% of mavacamten is eliminated in urine.[12][13] The mean elimination half-life is about 8 days.[17]
Administration
Available Dosage Forms and Strengths
Mavacamten is administered orally in capsule form, with available doses of 2.5 mg, 5 mg, 10 mg, and 15 mg.
Adult Dosages
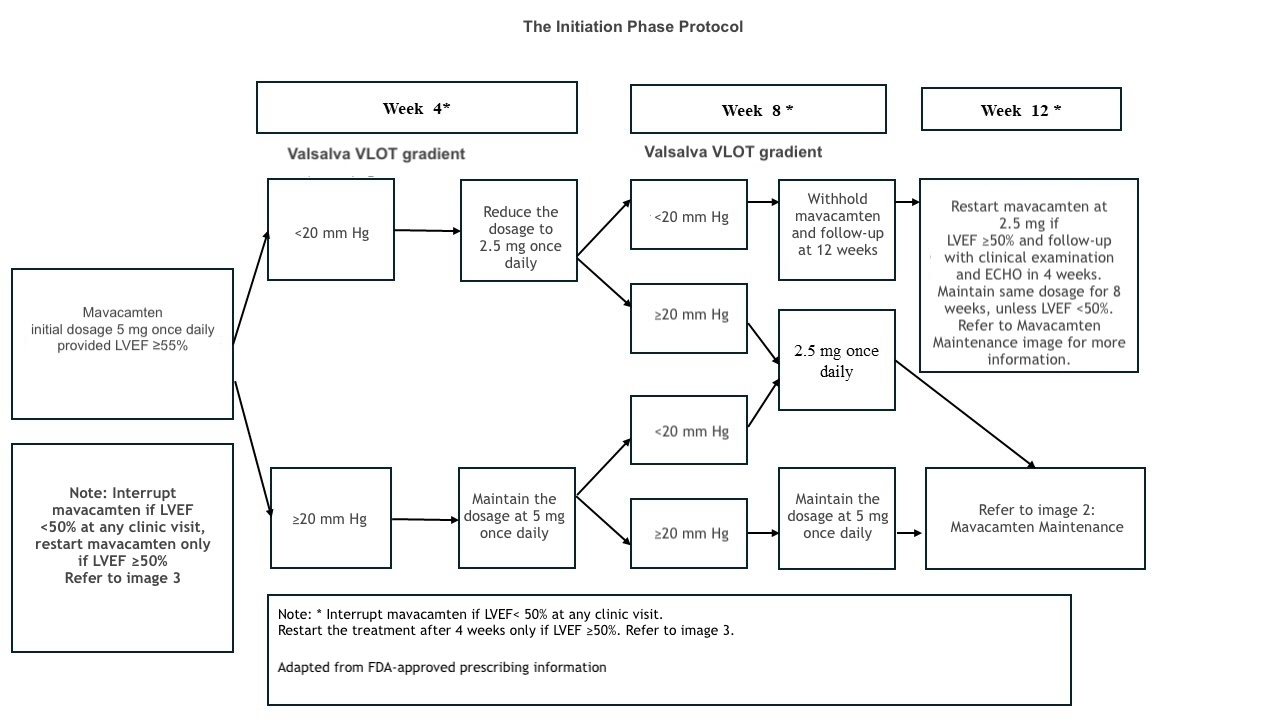
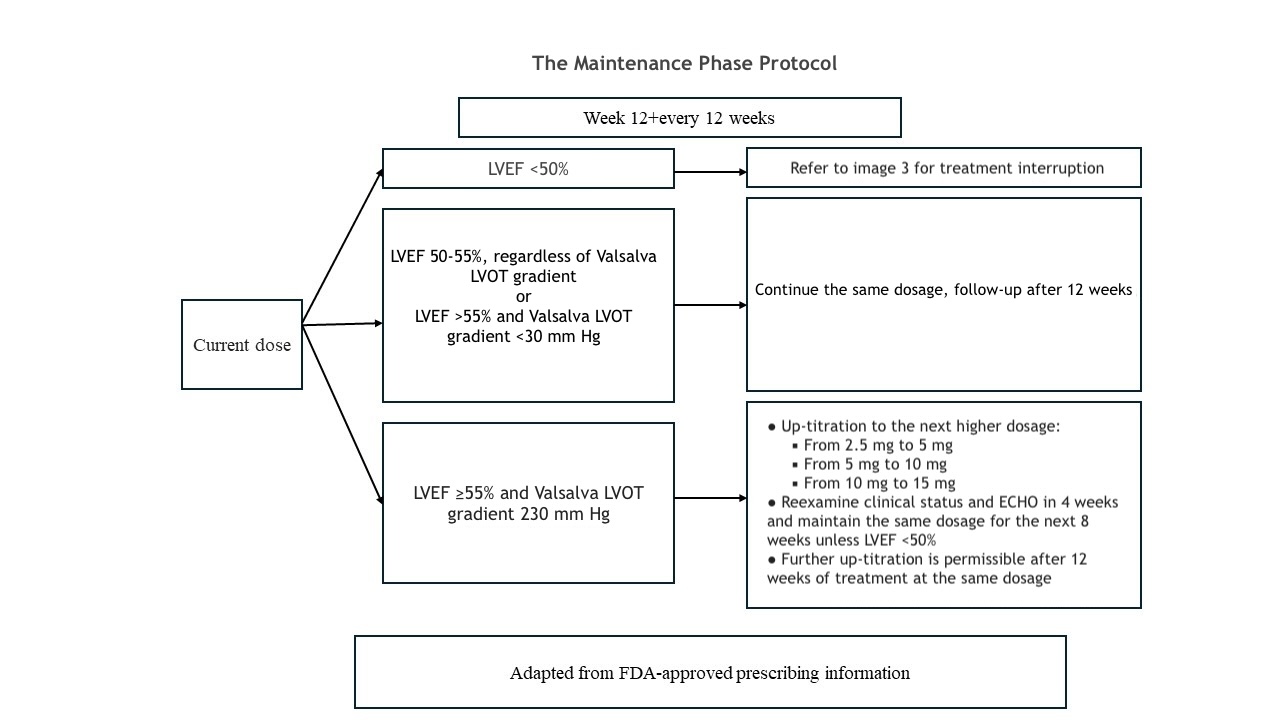
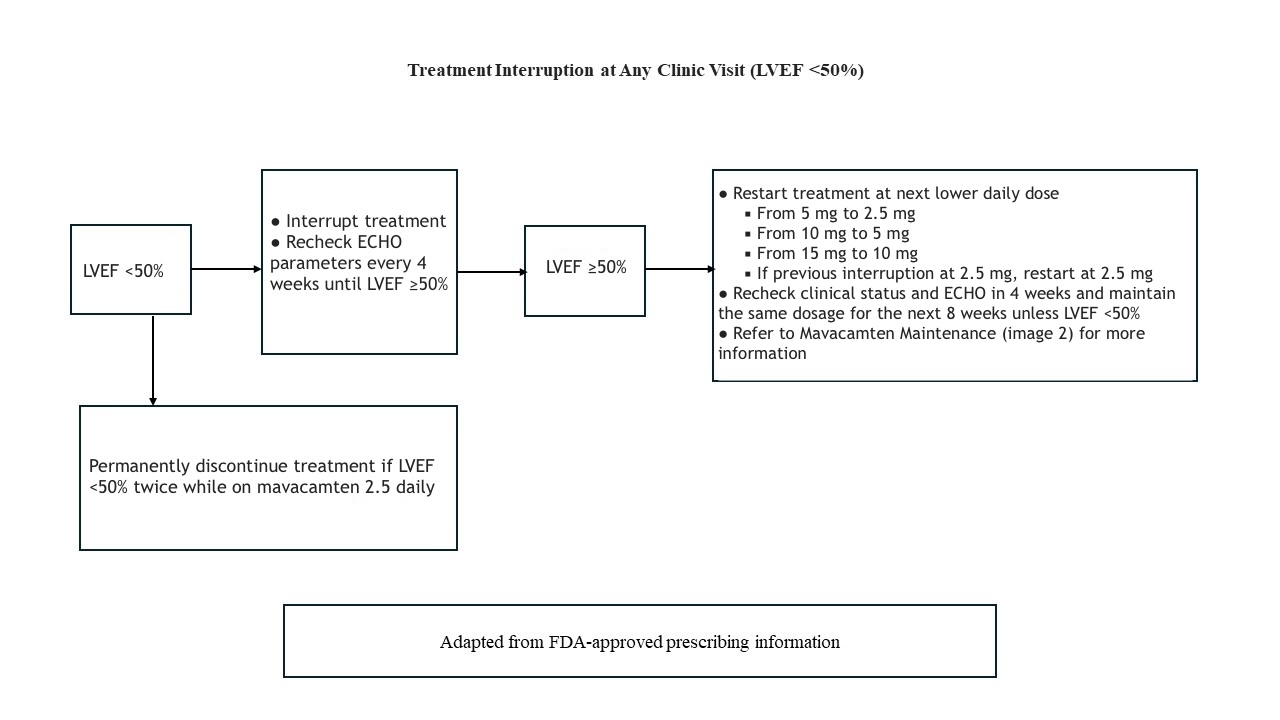
The recommended initial dosage of mavacamten is 5 mg once daily. Dose titration can occur every 4 weeks, with a maximum daily dose of 15 mg. The target plasma concentration ranges from 350 to 700 ng/mL, and it may take several weeks to reach steady-state levels and achieve the associated therapeutic effects.[17] The patient's clinical status, LVEF, and LVOT gradient should be assessed before starting treatment and monitored regularly during therapy. Please refer to the initial maintenance and treatment interruption protocol adapted from the prescribing information and the monitoring section for more details (see Images. Mavacamten Initiation, Mavacamten Maintenance, and Mavacamten Interruption).
Specific Patient Populations
Hepatic impairment: Mavacamten exposure (area under the curve [AUC]) significantly increases in patients with mild (Child-Pugh class A) or moderate (Child-Pugh class B) hepatic impairment. The impact of severe hepatic impairment (Child-Pugh class C) is unknown; therefore, mavacamten should be used cautiously in these patients.
Renal impairment: Dosage adjustments are not necessary for patients with mild-to-moderate renal impairment (estimated glomerular filtration rate [eGFR]: 60-89 mL/min/1.73 m2 and 30-59 mL/min/1.73 m2, respectively). However, the effects of severe renal impairment (eGFR: 15-30 mL/min/1.73 m2) and kidney failure (eGFR: <15 mL/min/1.73 m2, including patients on dialysis) remain uncertain.
Pregnancy considerations: Mavacamten was found to pose a risk of fetal toxicity based on animal studies. The absence of pregnancy should be confirmed before initiating treatment, and patients should be advised to use effective contraception during mavacamten therapy and for 4 months after treatment cessation. Combined hormonal contraceptives containing ethinyl estradiol and norethindrone are compatible with mavacamten. However, mavacamten may reduce the effectiveness of certain progestins. Therefore, it is recommended to use non-hormonal contraceptive methods alongside hormonal contraceptives during therapy and for 4 months after discontinuing mavacamten.[18]
Breastfeeding considerations: The presence of mavacamten in human or animal milk, its effects on the breastfed infant, and its impact on milk production are unknown. Therefore, when prescribing mavacamten to breastfeeding mothers, the benefits of breastfeeding should be weighed against the clinical necessity of the medication and the potential risks to the infant.
Pediatric patients: The safety and effectiveness of mavacamten have not been established for pediatric patients.
Older patients: Studies involving mavacamten found no significant differences in safety or effectiveness in older patients.
Adverse Effects
Mavacamten reduces systolic contraction and may lead to worsening heart failure or a complete block of ventricular function. A reduction in LVEF of up to 10% has been reported. In the phase III EXPLORER trial, dizziness (27%) and syncope (6%) were the most common adverse effects.[3][17]
The potential adverse reactions associated with mavacamten therapy include:
- Acute stress cardiomyopathy
- Ventricular tachycardia
- Angina pectoris
- Headache
- Dyspnea
- Chest pain
- Fatigue
- Palpitations
- Pedal edema
- Atrial fibrillation [19]
Drug-Drug Interactions
The concurrent administration of weak CYP2C19 inhibitors or moderate CYP3A4 inhibitors with mavacamten increases drug exposure. In patients on stable therapy with these inhibitors, mavacamten should be initiated at a daily dose of 5 mg. For patients already on mavacamten, the dose should be reduced (eg, from 15 to 10 mg) when starting these inhibitors. Weak CYP2C19 and moderate CYP3A4 inhibitors should be avoided in patients receiving 2.5 mg of mavacamten due to the lack of a lower dosage option.
Concurrent administration of mavacamten with medications that have adverse inotropic effects may exacerbate cardiac dysfunction. Coadministration with disopyramide, ranolazine, verapamil, β-blockers, or diltiazem should be avoided due to the increased risk of left ventricular systolic dysfunction and heart failure. Rigorous monitoring of LVEF is crucial when initiating or titrating negative inotropic agents until stable dosing and clinical response are achieved.
Contraindications
As mavacamten is metabolized by CYP2C19 and CYP3A4 enzymes, coadministration with potent CYP2C19 or CYP3A4 inhibitors (eg, verapamil and ketoconazole) or potent CYP2C19 or CYP3A4 inducers (eg, rifampin) is associated with a higher risk of systolic dysfunction, heart failure exacerbation, or loss of medication effectiveness.
Box Warnings
Mavacamten should not be initiated in patients with an LVEF of less than 55% due to the risk of worsening systolic function. The use of this medication is also not recommended for patients taking disopyramide, ranolazine, or for those using a non-dihydropyridine calcium channel blocker in combination with a β-blocker, as interactions with these medications have not been studied, and there is potential for additive negative inotropic effects.[3]
Mavacamten use in pregnant women is not recommended due to fetal toxicity observed in animal studies. Effective contraception should be ensured before initiating therapy. Mavacamten may reduce the effectiveness of combined hormonal contraceptives, so non-hormonal contraceptive methods not affected by CYP450 enzymes are recommended. Contraception should continue for at least 4 months after discontinuing mavacamten.
Mavacamten is available only through a Risk Evaluation and Mitigation Strategy (REMS) program due to the potential risk of heart failure.[20]
Monitoring
Patients on mavacamten require continuous monitoring for exacerbation of heart failure and periodic echocardiographic follow-up to assess LVEF and LVOT gradient. Mavacamten has been associated with nearly complete resolution of mitral valve systolic anterior motion and a reduction in the LVOT gradient. Additionally, reductions in left ventricular mass index, left atrial volume index, and lateral E/e' have been observed in patients receiving mavacamten. These echocardiographic improvements are also associated with reductions in natriuretic peptides and cardiac troponin I, which are markers of myocardial injury and stress. Regarding cardiac structural changes, mavacamten use has not been associated with reductions in interventricular septum thickness or left ventricular end-diastolic diameter. However, compared to placebo, significant changes have been observed in inferolateral wall thickness and left ventricular end-systolic diameter.[21]
Once treatment is started, the LVOT gradient should be assessed after 4 weeks. For patients with an LVOT gradient of less than 20 mm Hg, the dose should be titrated to the next lower available dose (eg, from 5 to 2.5 mg daily), with reevaluation at 12 weeks. The current dose should be maintained if the patient's LVOT gradient is between 20 and 30 mm Hg with an LVEF of more than 50%. If the LVOT gradient exceeds 20 mm Hg, a repeat evaluation is recommended at 8 weeks. For patients on 2.5 mg daily, if the LVOT gradient is between 20 and 30 mm Hg at the 8-week evaluation, continuing the same dose is advised.
For patients with an LVOT gradient of less than 20 mm Hg, treatment should be withheld, and reevaluation at week 12 is recommended.[22] To determine maintenance dosing, patients are assessed at week 12. The current mavacamten dose should be continued if the LVEF is 50% or greater and the LVOT gradient is less than 30 mm Hg. If the LVOT gradient exceeds 30 mm Hg, increasing to the next available dose and performing an echocardiographic evaluation at week 16 is recommended.[22]
Treatment should be discontinued if the patient's LVEF falls below 50%. Patients who experience severe infections requiring hospitalization or uncontrolled tachyarrhythmia are at higher risk of systolic dysfunction and should be evaluated to determine whether to continue or interrupt treatment. After discontinuation, an echocardiographic evaluation should be performed 4 weeks later to reassess the LVEF and LVOT gradient. When reinitiating mavacamten, the dose should be reduced to the next lower dose (eg, from 10 to 5 mg daily). Repeat evaluations are recommended at weeks 4 and 8 after treatment is restarted. If the patient's LVEF falls below 50% again, permanent discontinuation of mavacamten is advised.[22]
Toxicity
Signs and Symptoms of Overdose
Mavacamten overdose is associated with systolic dysfunction, which can manifest as worsening functional capacity and exacerbation of heart failure. Although no carcinogenic or mutagenic effects have been linked to mavacamten, animal studies have shown QT prolongation and cardiac osseous metaplasia in subjects receiving 10 mg/kg/d.[3][22]
Management of Overdose
An antidote for mavacamten does not exist. In cases of overdose, mavacamten should be discontinued immediately, and efforts should be made to maintain hemodynamic stability while closely monitoring left ventricular function. Activated charcoal can effectively reduce mavacamten absorption if administered within 2 hours of ingestion; however, it does not affect exposure levels if given 6 hours after dosing.[23] A poison control center or medical toxicologist should be contacted for updated recommendations.
Enhancing Healthcare Team Outcomes
Providing quality care for patients with heart failure necessitates a multidisciplinary and interprofessional team, including cardiologists, critical care and primary care clinicians, registered nurses, pharmacists, social workers, physical therapists, and rehabilitation staff. Before 2022, treatment for patients with obstructive HCM was primarily aimed at symptomatic relief through pharmacologic treatment or interventional approaches such as SRTs. Mavacamten is a first-in-class cardiac myosin inhibitor developed to target the underlying pathophysiology of obstructive HCM directly.[24]
Interprofessional strategies should be tailored to enhance patient outcomes. The prescribing clinician may consult with a cardiology-specialized pharmacist to determine the appropriateness of mavacamten for the patient and address potential drug-drug interactions collaboratively. Nurses are responsible for patient counseling and answering questions about the medication, with pharmacists reinforcing these points during dispensing. Any interprofessional healthcare team member who observes a significant change in the patient should promptly communicate with other team members to ensure coordinated, optimal patient care and keep everyone updated on the patient's status. Proper notations should be made in the patient's record. Therapists must also report any changes in the patient's condition to facilitate necessary adjustments in the treatment plan.
Mavacamten therapy can enhance the patient's NYHA functional class and improve various hemodynamic and structural parameters. Approved by the FDA for patients with obstructive HCM and symptomatic heart failure, mavacamten represents a significant therapeutic advance. The FDA has designated mavacamten as a 'breakthrough therapy.'[25] A thorough understanding of mavacamten's pharmacological and cardiovascular effects is essential for effective treatment guidance, monitoring, and adjustment in patients with obstructive HCM. Ongoing trials also evaluate mavacamten's safety and efficacy in patients with nonobstructive HCM or those with obstructive HCM who qualify for interventional therapy. An interprofessional team approach, with effective communication among clinicians, cardiologists, pharmacists, and nurses, is crucial for minimizing potential adverse effects, improving disease progression and quality of life, and enhancing patient outcomes.
Media
References
Geske JB, Ommen SR, Gersh BJ. Hypertrophic Cardiomyopathy: Clinical Update. JACC. Heart failure. 2018 May:6(5):364-375. doi: 10.1016/j.jchf.2018.02.010. Epub 2018 Apr 11 [PubMed PMID: 29655825]
Sparrow AJ, Watkins H, Daniels MJ, Redwood C, Robinson P. Mavacamten rescues increased myofilament calcium sensitivity and dysregulation of Ca(2+) flux caused by thin filament hypertrophic cardiomyopathy mutations. American journal of physiology. Heart and circulatory physiology. 2020 Mar 1:318(3):H715-H722. doi: 10.1152/ajpheart.00023.2020. Epub 2020 Feb 21 [PubMed PMID: 32083971]
Olivotto I, Oreziak A, Barriales-Villa R, Abraham TP, Masri A, Garcia-Pavia P, Saberi S, Lakdawala NK, Wheeler MT, Owens A, Kubanek M, Wojakowski W, Jensen MK, Gimeno-Blanes J, Afshar K, Myers J, Hegde SM, Solomon SD, Sehnert AJ, Zhang D, Li W, Bhattacharya M, Edelberg JM, Waldman CB, Lester SJ, Wang A, Ho CY, Jacoby D, EXPLORER-HCM study investigators. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (London, England). 2020 Sep 12:396(10253):759-769. doi: 10.1016/S0140-6736(20)31792-X. Epub 2020 Aug 29 [PubMed PMID: 32871100]
Level 1 (high-level) evidenceXie J, Wang Y, Xu Y, Fine JT, Lam J, Garrison LP. Assessing health-related quality-of-life in patients with symptomatic obstructive hypertrophic cardiomyopathy: EQ-5D-based utilities in the EXPLORER-HCM trial. Journal of medical economics. 2022 Jan-Dec:25(1):51-58. doi: 10.1080/13696998.2021.2011301. Epub [PubMed PMID: 34907813]
Level 2 (mid-level) evidenceSaberi S, Cardim N, Yamani M, Schulz-Menger J, Li W, Florea V, Sehnert AJ, Kwong RY, Jerosch-Herold M, Masri A, Owens A, Lakdawala NK, Kramer CM, Sherrid M, Seidler T, Wang A, Sedaghat-Hamedani F, Meder B, Havakuk O, Jacoby D. Mavacamten Favorably Impacts Cardiac Structure in Obstructive Hypertrophic Cardiomyopathy: EXPLORER-HCM Cardiac Magnetic Resonance Substudy Analysis. Circulation. 2021 Feb 9:143(6):606-608. doi: 10.1161/CIRCULATIONAHA.120.052359. Epub 2020 Nov 15 [PubMed PMID: 33190524]
Desai N, Xie J, Wang Y, Sutton MB, Whang J, Fine JT, Garrison LP Jr. Projecting the Long-term Clinical Value of Mavacamten for the Treatment of Obstructive Hypertrophic Cardiomyopathy in the United States: An Assessment of Net Health Benefit. Clinical therapeutics. 2022 Jan:44(1):52-66.e2. doi: 10.1016/j.clinthera.2021.11.006. Epub 2021 Dec 12 [PubMed PMID: 34911641]
Ho CY, Mealiffe ME, Bach RG, Bhattacharya M, Choudhury L, Edelberg JM, Hegde SM, Jacoby D, Lakdawala NK, Lester SJ, Ma Y, Marian AJ, Nagueh SF, Owens A, Rader F, Saberi S, Sehnert AJ, Sherrid MV, Solomon SD, Wang A, Wever-Pinzon O, Wong TC, Heitner SB. Evaluation of Mavacamten in Symptomatic Patients With Nonobstructive Hypertrophic Cardiomyopathy. Journal of the American College of Cardiology. 2020 Jun 2:75(21):2649-2660. doi: 10.1016/j.jacc.2020.03.064. Epub [PubMed PMID: 32466879]
Desai MY, Wolski K, Owens A, Naidu SS, Geske JB, Smedira NG, Schaff H, Lampl K, McErlean E, Sewell C, Zhang D, Edelberg JM, Sehnert AJ, Nissen SE. Study design and rationale of VALOR-HCM: evaluation of mavacamten in adults with symptomatic obstructive hypertrophic cardiomyopathy who are eligible for septal reduction therapy. American heart journal. 2021 Sep:239():80-89. doi: 10.1016/j.ahj.2021.05.007. Epub 2021 May 24 [PubMed PMID: 34038706]
Desai MY, Owens A, Geske JB, Wolski K, Naidu SS, Smedira NG, Cremer PC, Schaff H, McErlean E, Sewell C, Li W, Sterling L, Lampl K, Edelberg JM, Sehnert AJ, Nissen SE. Myosin Inhibition in Patients With Obstructive Hypertrophic Cardiomyopathy Referred for Septal Reduction Therapy. Journal of the American College of Cardiology. 2022 Jul 12:80(2):95-108. doi: 10.1016/j.jacc.2022.04.048. Epub [PubMed PMID: 35798455]
Bishev D, Fabara S, Loseke I, Alok A, Al-Ani H, Bazikian Y. Efficacy and Safety of Mavacamten in the Treatment of Hypertrophic Cardiomyopathy: A Systematic Review. Heart, lung & circulation. 2023 Sep:32(9):1049-1056. doi: 10.1016/j.hlc.2023.05.019. Epub 2023 Jul 14 [PubMed PMID: 37453852]
Level 1 (high-level) evidenceWriting Committee Members, Ommen SR, Ho CY, Asif IM, Balaji S, Burke MA, Day SM, Dearani JA, Epps KC, Evanovich L, Ferrari VA, Joglar JA, Khan SS, Kim JJ, Kittleson MM, Krittanawong C, Martinez MW, Mital S, Naidu SS, Saberi S, Semsarian C, Times S, Waldman CB. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR Guideline for the Management of Hypertrophic Cardiomyopathy: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Journal of the American College of Cardiology. 2024 Jun 11:83(23):2324-2405. doi: 10.1016/j.jacc.2024.02.014. Epub 2024 May 8 [PubMed PMID: 38727647]
Level 1 (high-level) evidenceGrillo MP, Erve JCL, Dick R, Driscoll JP, Haste N, Markova S, Brun P, Carlson TJ, Evanchik M. In vitro and in vivo pharmacokinetic characterization of mavacamten, a first-in-class small molecule allosteric modulator of beta cardiac myosin. Xenobiotica; the fate of foreign compounds in biological systems. 2019 Jun:49(6):718-733. doi: 10.1080/00498254.2018.1495856. Epub 2018 Oct 1 [PubMed PMID: 30044681]
Anderson RL, Trivedi DV, Sarkar SS, Henze M, Ma W, Gong H, Rogers CS, Gorham JM, Wong FL, Morck MM, Seidman JG, Ruppel KM, Irving TC, Cooke R, Green EM, Spudich JA. Deciphering the super relaxed state of human β-cardiac myosin and the mode of action of mavacamten from myosin molecules to muscle fibers. Proceedings of the National Academy of Sciences of the United States of America. 2018 Aug 28:115(35):E8143-E8152. doi: 10.1073/pnas.1809540115. Epub 2018 Aug 13 [PubMed PMID: 30104387]
Keam SJ. Mavacamten: First Approval. Drugs. 2022 Jul:82(10):1127-1135. doi: 10.1007/s40265-022-01739-7. Epub 2022 Jul 8 [PubMed PMID: 35802255]
Li Q, Liu YN, Chen C, Xu RA, Xie S, Zhan R. Effects of CYP2C19 inhibitors on mavacamten pharmacokinetics in rats based on UPLC-MS/MS. Chemico-biological interactions. 2023 Aug 1:380():110531. doi: 10.1016/j.cbi.2023.110531. Epub 2023 May 6 [PubMed PMID: 37150496]
Chiang M, Sychterz C, Perera V, Merali S, Palmisano M, Templeton IE, Gaohua L. Physiologically Based Pharmacokinetic Modeling and Simulation of Mavacamten Exposure with Drug-Drug Interactions from CYP Inducers and Inhibitors by CYP2C19 Phenotype. Clinical pharmacology and therapeutics. 2023 Oct:114(4):922-932. doi: 10.1002/cpt.3005. Epub 2023 Aug 6 [PubMed PMID: 37467157]
Heitner SB, Jacoby D, Lester SJ, Owens A, Wang A, Zhang D, Lambing J, Lee J, Semigran M, Sehnert AJ. Mavacamten Treatment for Obstructive Hypertrophic Cardiomyopathy: A Clinical Trial. Annals of internal medicine. 2019 Jun 4:170(11):741-748. doi: 10.7326/M18-3016. Epub 2019 Apr 30 [PubMed PMID: 31035291]
Chiang M, Sychterz C, Gaohua L, Perera V, Gretler DD, Florea V, Merali S. Drug-Drug Interaction Potential of Mavacamten with Oral Contraceptives: Results from a Clinical Pharmacokinetic Study and a Physiologically Based Pharmacokinetic Model. Journal of clinical pharmacology. 2023 Nov:63(11):1275-1282. doi: 10.1002/jcph.2298. Epub 2023 Jul 26 [PubMed PMID: 37376778]
Rader F, Oręziak A, Choudhury L, Saberi S, Fermin D, Wheeler MT, Abraham TP, Garcia-Pavia P, Zwas DR, Masri A, Owens A, Hegde SM, Seidler T, Fox S, Balaratnam G, Sehnert AJ, Olivotto I. Mavacamten Treatment for Symptomatic Obstructive Hypertrophic Cardiomyopathy: Interim Results From the MAVA-LTE Study, EXPLORER-LTE Cohort. JACC. Heart failure. 2024 Jan:12(1):164-177. doi: 10.1016/j.jchf.2023.09.028. Epub [PubMed PMID: 38176782]
Woodland M, Al-Horani RA. New Era: Mavacamten for Obstructive Hypertrophic Cardiomyopathy. Cardiovascular & hematological agents in medicinal chemistry. 2023:21(2):78-83. doi: 10.2174/1871525721666221019095218. Epub [PubMed PMID: 36278454]
Hegde SM, Lester SJ, Solomon SD, Michels M, Elliott PM, Nagueh SF, Choudhury L, Zemanek D, Zwas DR, Jacoby D, Wang A, Ho CY, Li W, Sehnert AJ, Olivotto I, Abraham TP. Effect of Mavacamten on Echocardiographic Features in Symptomatic Patients With Obstructive Hypertrophic Cardiomyopathy. Journal of the American College of Cardiology. 2021 Dec 21:78(25):2518-2532. doi: 10.1016/j.jacc.2021.09.1381. Epub [PubMed PMID: 34915982]
Ho CY, Olivotto I, Jacoby D, Lester SJ, Roe M, Wang A, Waldman CB, Zhang D, Sehnert AJ, Heitner SB. Study Design and Rationale of EXPLORER-HCM: Evaluation of Mavacamten in Adults With Symptomatic Obstructive Hypertrophic Cardiomyopathy. Circulation. Heart failure. 2020 Jun:13(6):e006853. doi: 10.1161/CIRCHEARTFAILURE.120.006853. Epub 2020 Jun 5 [PubMed PMID: 32498620]
Merali S, Chiang M, Sychterz C, Chao L, Simmons T, Xu Y, Zhao A, Attanasio M, Murthy B, Perera V. Effect of Activated Charcoal on Mavacamten Pharmacokinetics in Healthy Participants. American journal of cardiovascular drugs : drugs, devices, and other interventions. 2024 Jul:24(4):569-575. doi: 10.1007/s40256-024-00659-z. Epub 2024 Jun 26 [PubMed PMID: 38926266]
Liao HL, Liang Y, Liang B. Evaluation of mavacamten in patients with hypertrophic cardiomyopathy. Journal of cardiovascular medicine (Hagerstown, Md.). 2024 Jul 1:25(7):491-498. doi: 10.2459/JCM.0000000000001638. Epub 2024 May 29 [PubMed PMID: 38814051]
Braunwald E, Saberi S, Abraham TP, Elliott PM, Olivotto I. Mavacamten: a first-in-class myosin inhibitor for obstructive hypertrophic cardiomyopathy. European heart journal. 2023 Nov 21:44(44):4622-4633. doi: 10.1093/eurheartj/ehad637. Epub [PubMed PMID: 37804245]