Introduction
Mycobacterium tuberculosis is responsible for 5.9% of community-acquired central nervous system (CNS) infections worldwide.[1] Neurological or CNS tuberculosis (CNS-TB) may take 1 of 3 clinicopathological forms:
- Tubercular meningitis (TBM), affecting the CNS diffusely
- CNS tuberculoma, the focal type
- Spinal arachnoiditis, also called "tuberculous radiculomyelitis" (TBRM), involving only the spine [2]
Of these forms, TBM predominates as it causes 70% to 80% of CNS-TB infections. TBM presents with subacute-to-chronic meningitis signs and symptoms, with disease severity commensurate with illness duration.[3] Diagnosis is fraught with challenges and is often delayed due to the varied and nonspecific presentations.[4] Besides the clinical clues, diagnostic indicators in cerebrospinal fluid (CSF) include mononuclear pleocytosis, low sugar values, and high protein concentrations. Identifying Mycobacterium tuberculosis (MTB) in CSF by staining, culture methods, and molecular analysis is confirmatory but may be challenging.[5]
Advanced radiological imaging techniques are usually of great assistance in making presumptive diagnoses. Vasculitic infarcts, cranial nerve (CN) palsies, multiple neurological deficits, and hydrocephalus frequently complicate CNS-TB. A strong clinical suspicion is typically enough to start prompt antitubercular therapy. The 4-drug regimen of isoniazid, rifampin, pyrazinamide, and ethambutol with adjunctive corticosteroid reduces morbidity and mortality. However, CNS-TB diagnosis and management may be complicated by drug resistance, immune reconstitution inflammatory syndrome (IRIS), and HIV coinfection.
Treatment efficacy depends upon the timing. Multiple factors determine the prognosis, the most important being the TBM clinical stage at initial presentation. Untreated or unrecognized TBM may cause death within 5 to 8 weeks of disease onset.
Normal Protective Barriers of the Central Nervous System
The CNS is shielded from potentially harmful blood-borne bacteria by 2 vascular barriers: the blood-brain barrier (BBB) and blood-CSF barrier (BCSFB). The BBB, primarily consisting of brain microvascular endothelial cells, regulates the passage of substances between the blood and CNS with tight junctions and specialized transport mechanisms. Supporting elements like pericytes and astrocytes also play crucial roles in maintaining BBB integrity. In contrast, the BCSFB is formed by choroid plexus epithelial cells and the arachnoid membrane, similarly employing tight junctions to control the exchange between blood and CSF.
However, in vitro and animal models have shown that MTB can invade brain endothelial cells by rearranging cellular actin molecules. The MTB gene Rv0931c is implicated in promoting CNS infection by facilitating bacillary endothelial adhesion. MTB may also exploit the "Trojan horse" mechanism, utilizing infected macrophages and neutrophils to traverse the BBB.[6]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
MTB is the organism responsible for CNS-TB. This alcohol and acid-fast bacillus may be visualized microscopically using the Ziehl-Neelsen, auramine-rhodamine, and Kinyoun stains. The etiological contribution of the other 4 Mycobacterium species in causing CNS-TB has not been established. Humans are the only known MTB hosts or reservoirs. Infection is primarily acquired through airborne aerosols from infected individuals.
Epidemiology
Around 25% to 30% of the human population is infected with MTB. CNS-TB is reported in 1% to 2% of individuals with active tuberculosis.[7] CNS-TB accounts for 5% to 8% of extrapulmonary tuberculosis (EPTB) cases in immunocompetent hosts. Approximately 70% to 80% of CNS-TB cases present as TBM.[8]
Tuberculomas are seen mostly in India and parts of Asia and may account for 20% to 30% of all intracranial space-occupying lesions.[9] An estimated 9,272 CNS-TB cases were diagnosed in the U.S. in 2016.[10] Since 2010, the incidence of tuberculosis has fallen by 2% to 3% every year, especially in 2020, probably due to the pandemic mitigation efforts, travel restrictions, or delayed or missed tuberculosis diagnoses.[11] However, the incidence of reported tuberculosis rose by 9.4% between 2020 and 2021.[12] Despite the diagnostic and treatment advances, CNS–TB still carries a mortality rate of 15% to 40%, especially in children.[13]
Pathophysiology
The 3 CNS-TB forms develop by different mechanisms. Understanding each condition's pathophysiology, explained below, is key to diagnosis.
Tuberculous Meningitis
CNS-TB may arise from hematogenous MTB dissemination following a primary lung infection (pulmonary tuberculosis or PTB) or late disease reactivation.[14] Meningeal or ependymal seeding results from this spread, forming multiple small granulomatous foci or tubercles (Rich foci) that may proliferate, coalesce, caseate, and rupture into the subarachnoid space before the onset of meningitis. Free bacilli produce an intense, cytokine-mediated host inflammatory response, leading to the following:
- Leptomeningitis, ependymitis, choroid plexitis, encephalitis, and pachymeningitis with basal and opticochiasmatic arachnoiditis (OCA)
- Vasculitis with endarteritis and infarcts
- Hydrocephalus secondary to disturbed CSF flow and absorption
The extensive exudative arachnoiditis at the brain's base and the optic chiasma entraps multiple CNs in the area, including the abducens, optic, oculomotor, and trochlear. Arteritis and phlebitis result in vasculitic infarcts due to arterial vasospasm, thrombosis, or hemorrhage.[15] The disparate inflammatory response in individuals with TBM is thought to be due to human genetic polymorphisms in the leukotriene A4 hydrolase gene and toll-interleukin-1 receptor domain.[16]
Inflammation can spread to the basal cisterns in many patients symptomatic for at least 3 weeks, disrupting CSF circulation and absorption and producing communicating hydrocephalus[17] Obstructive hydrocephalus occurs, though rarely, due to ependymitis-induced narrowing of the cerebral aqueduct or compression by brainstem tuberculoma in the 4th ventricle.
Tuberculoma
Deep-seated tubercular granulomatous foci acquired during initial bacteremia may coalesce and develop into conglomerated caseous masses called "tuberculomas" without producing meningitis. Often, tuberculomas may be present as clinically silent single or multiple lesions in patients with TBM and detected on brain imaging. Tuberculomas may form tubercular abscesses in immunosuppressed individuals. Spinal cord tuberculoma may form at any cord level through similar mechanisms or by local extension from nearby tubercular spondylitis.
Spinal Arachnoiditis
Spinal arachnoiditis may arise as a result of previous TBM or vertebral osteomyelitis. The caseating inflammation may progress over weeks to months, leading to nerve root clumping or cord encasement (partial/total) by the thick gelatinous exudate or fibrous mass.
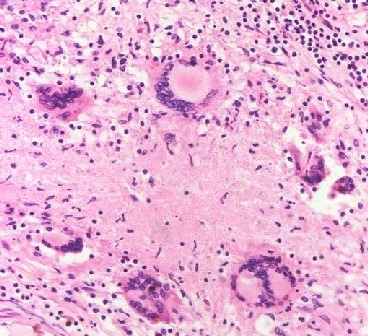
Histopathology
Histologically, the thick inflammatory meningeal exudate is granulomatous, with epithelioid macrophages, Langhans' giant cells, lymphocytes, plasma cells, and fibroblasts (see Image. Tuberculous Meningitis Histopathology). Tuberculomas are bigger encapsulated epithelioid granulomas with similar pleocytosis arranged in layers but a necrotic caseous center without pus. A tuberculous abscess has a thick wall with a liquefied pus core containing viable MTB bacilli.
History and Physical
The different CNS-TB forms produce various manifestations. The historical and physical features of each type are explained below.
Tuberculous Meningitis
Patients with TBM often present with a subacute, progressive febrile illness beginning with a prodrome of constitutional symptoms, including lassitude, malaise, night sweats, and intermittent headaches. A well-defined meningitic phase is observed in the following fortnight or more. This phase includes protracted headaches, vomiting, personality changes, confusion, and meningismus symptoms. Patients with TBM may deteriorate rapidly, developing varying degrees of confusion and coma. Seizures, multiple CN deficits, focal neurological impairments, and stroke syndromes may supervene.
Children may have prominent irritability, vomiting, and focal or generalized convulsions. Individuals with untreated TBM may not survive beyond 5 to 8 weeks of illness. CN palsies are seen in 20% to 30% of patients with TBM, mostly involving CN VI.[18] OCA with blindness is a significant TBM complication.[19]
Tuberculoma
This CNS-TB manifestation should be included in the differential diagnosis of space-occupying lesions in young individuals. The usual symptoms include headaches, seizures, progressive neurological deficits, and papilledema with or without meningitis.
Spinal Arachnoiditis
Individuals with this condition present with radiculomyelopathy secondary to nerve root entrapment and cord encasement due to severe arachnoiditis at single or multiple levels. Patients may complain of radicular pain, hyperesthesia, flaccid paralysis, and urinary or stool incontinence. Cord infarction may occur due to anterior spinal arteritis and thrombosis (see Image. Pott Spine).
Atypical Presentations
CNS-TB may manifest in 10% to 30% of adult patients with miliary tuberculosis. The condition arises from hematogenous MTB dissemination, with immunocompromised patients being the most vulnerable. The brain parenchyma may show multiple scattered tiny granulomatous foci, which may or may not show ring enhancement.[20]
TBM may also manifest as progressive cognitive dysfunction or psychosis in rare cases. The condition may likewise mimic acute encephalitis or typical pyogenic meningitis. Atypical cases may present with florid cranial polyneuropathy or hydrocephalus complications that precede meningitis signs.[21]
Tuberculous Meningitis and HIV Infection
HIV coinfection does not affect CNS-TB's clinical manifestations, CSF picture, or prognosis. However, HIV-seropositive TBM may have a normal CSF, with radiographic clues holding greater importance in such cases. Cryptococcal meningitis is a close differential diagnosis and may sometimes occur concurrently. Intracerebral tuberculomas are more likely to occur in HIV patients than in the general population, often as multiloculated tubercular abscesses. CNS lymphoma or toxoplasma encephalitis closely mimics the condition. However, cisternal enhancement, basal ganglia infarction, and communicating hydrocephalus favor a CNS-TB diagnosis.[22]
Evaluation
Clinical Diagnosis of Tuberculous Meningitis
CNS-TB is a paucibacillary disease, often eluding definitive diagnosis.[23] Conventional diagnostic techniques are insensitive and laborious.[24] Clinical scoring algorithms for definitive CNS-TB diagnosis are flawed and unreliable.[25][26] With early and precise identification being crucial for survival, diagnosis typically relies on a combination of evidence from patient history, neurological examination, CSF analysis, and radiological imaging.[27]
Newer molecular techniques help confirm the presence of MTB in CSF, which is often challenging. Still, a definitive diagnosis is not always achievable, necessitating presumptive and prompt treatment initiation to mitigate delay-related risks. Investigating epidemiological factors and personal risk variables is crucial, even though such data may be limited in adults. Notably, active tuberculosis exposure can be identified in 70% to 90% of children with TBM.
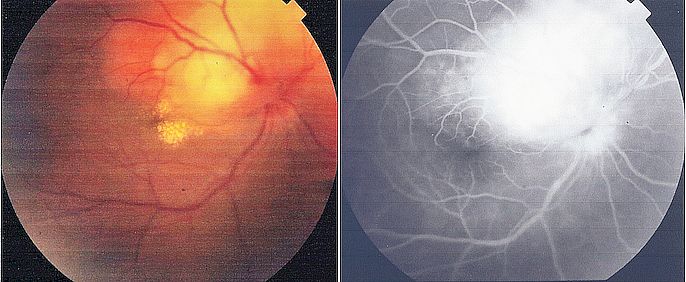
Identifying active extraneural infection occasionally aids in determining a presumptive diagnosis. Chest radiographs are beneficial in most pediatric TBM cases and approximately 50% of adult cases (see Image. Pulmonary Tuberculosis). Laboratory analysis may indicate mild anemia, lymphocytosis, and hyponatremia. A positive tuberculin test offers supportive evidence, notably in children, though a negative result does not rule out the diagnosis. Funduscopy may also uncover papilledema and choroid tubercles, aiding diagnosis (see Image. Tuberculoma on Funduscopy).[28]
Cerebrospinal Fluid Examination and Culture
A pellicle or cobweb may form during gross CSF examination, which is representative but not pathognomonic of TBM. A xanthochromic CSF indicates unusually high protein content. CSF cytological analysis typically shows the total cell count between 100 and 500/mm3 in the majority. Less than 100 cells/mm3 are present in 15% of cases. Pleocytosis may be absent in people of advanced age or with miliary tuberculosis-related CNS-TB or HIV coinfection.
The CSF cell number may be 500 to 1,500 cells/mm3 in 20% of cases. A transient polymorphonuclear predominance or mixed pleocytosis may also be observed in the first week of illness. However, predominant lymphomononuclear pleocytosis is the typical CSF finding in TBM. CSF protein concentration varies from 100 to 500 mg/dl. About 25% of cases may have less than 100 mg/dl of protein, and 10% may have greater than 500 mg/dl. Values exceeding 1 g/dl is characteristic of severe adhesive spinal arachnoiditis and portends a poor prognosis. The CSF glucose level is less than 45 mg/dl in 80% of cases. However, unlike acute pyogenic meningitis, CSF glucose is never undetectable.
Microbiological Tests
Demonstrating MTB in the CSF smear using Ziehl-Neelsen and auramine-rhodamine staining is crucial but elusive. The detection rates range from 12.5% to 69%, showing significant variability across reports. Traditional Lowenstein-Jensen culture takes 4 to 8 weeks for growth reporting but detects MTB only in 25% to 70% of cases. Liquid culture media like Septi-Chek/Middlebrook 7H9 enhance detection rates. Detection often improves with larger sample volumes, more CSF specimens, centrifugation, and preparing thick smears from the pellicle.[29]
Molecular Diagnostic Techniques
The World Health Organization recommends Xpert MTB/rifampin (Xpert MTB/RIF), a polymerase chain reaction technique, to be used for molecular TBM diagnosis in CSF specimens, particularly in situations with limited sample volume.[30][31] This quick and automated cartridge-based nucleic acid amplification test targets MTB's rpob gene, enabling simultaneous detection of MTB and rifampin resistance.[32] An Xpert MTB/RIF meta-analysis demonstrated a sensitivity of 80.5% and a specificity of 97.8%.[33] Loop-mediated isothermal amplification assay is another promising, rapid, and cost-effective technique in resource-limited regions.[34]
CSF assays for the MTB cell wall glycolipid lipoarabinomannan show sensitivity comparable to Xpert MTB/RIF, potentially aiding TBM diagnosis in patients with HIV, particularly in resource-limited settings.[35] Lipoarabinomannan detection in CSF demonstrates high TBM specificity.[36] CSF interferon-γ release assays have superior diagnostic accuracy over blood interferon-γ release assays in evaluating TBM.[37][38]
Metagenomic next-generation sequencing (mNGS) has recently been shown to provide rapid and sensitive diagnostic capability in TBM.[39][40] Adding mNGS to CSF analysis may prove to be a valuable diagnostic tool when combined with traditional diagnostic methods.
Adenosine Deaminase
Adenosine deaminase (ADA) is an enzyme produced by T-lymphocytes that serves as an indirect measure of the host response to MTB and holds significant diagnostic value. Elevated ADA levels are observed in the CSF of 60% to 100% of individuals with TBM, with a cutoff value of 9.5 U/L used to distinguish TBM from non-TBM cases. Dynamic CSF-ADA activity monitoring may provide further diagnostic assistance.[41] One study demonstrates that ADA measurement has a sensitivity of 92.5% and a specificity of 97%, with the cutoff set to 10 U/L.[42]
Imaging
Neuroimaging is vital in diagnosing suspected CNS-TB cases, helping ensure a timely and correct diagnosis. Neuroimaging ideally must include the entire neuroaxis. The evaluation modalities include brain computed tomography (CT) or magnetic resonance imaging, with contrast-enhanced MRI having a superior delineating ability. Newer techniques further enhance MRI's diagnostic reliability in atypical or difficult cases (see Image. Tuberculous Meningitis on Magnetic Resonance Imaging).[43]
The diagnostic triad of TBM consists of the following:
- Extensive basilar leptomeningeal enhancement and exudates, found in 38% to 89%
- Hydrocephalus, detected in 60% to 75%
- Cerebral infarcts, seen in 15% to 28%, followed by tuberculoma in another 27% of cases [44][45][46]
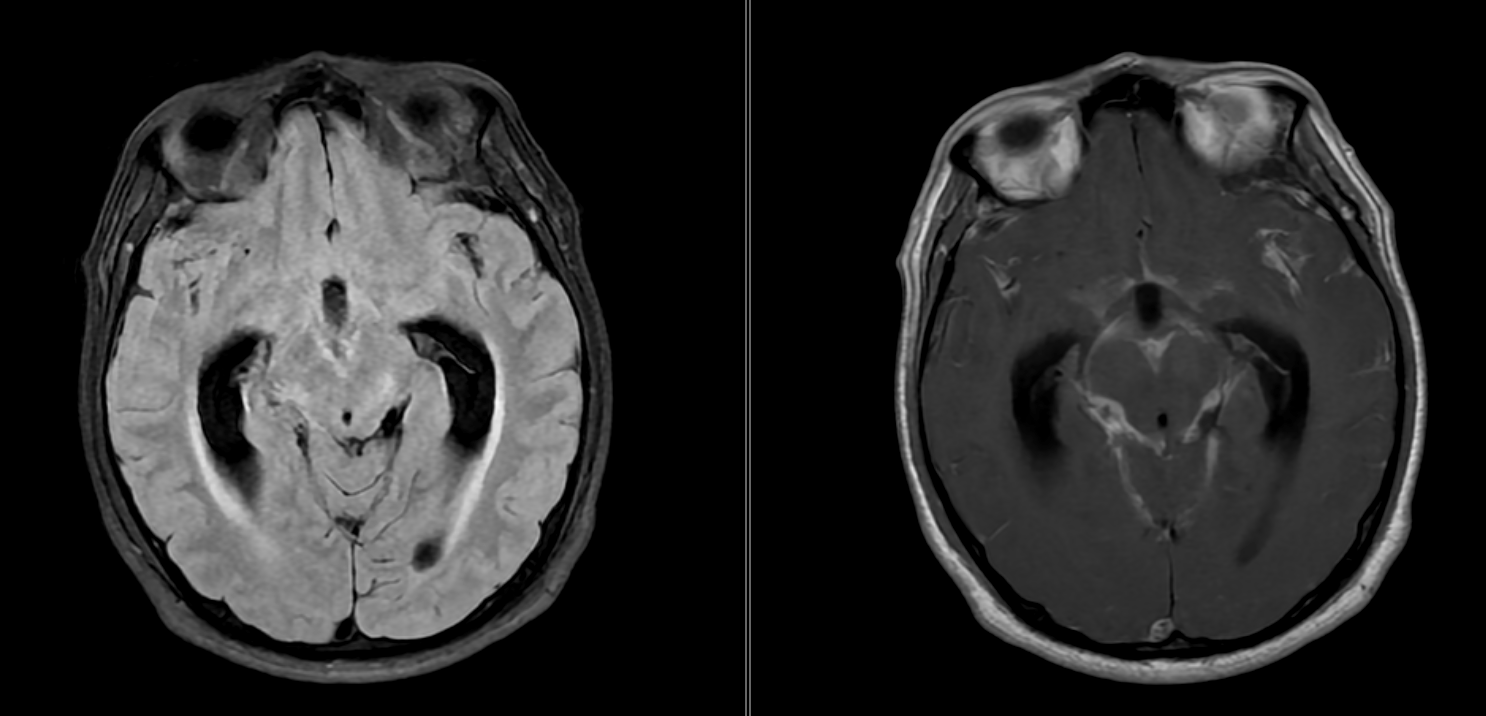
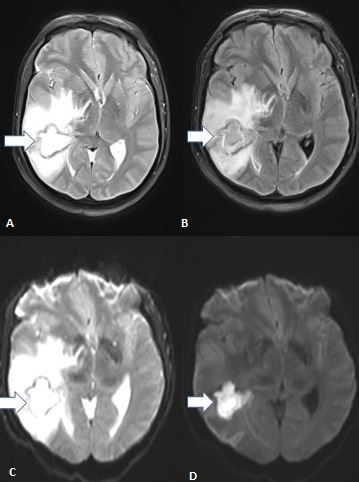
TBM exudates have certain areas of predilection. Optochiasmatic arachnoiditis is almost pathognomonic of TBM.[47] The other areas include the interpeduncular fossa, perimesencephalic region, cisterna ambiens, and suprasellar and Sylvian cisterns. Exudates are best discerned on MRI fluid-attenuated inversion recovery sequences, while enhanced meninges are more visible on postcontrast spin echo MRI (see Image. Tuberculous Meningitis on MRI).
On CT, intense basal cisternal enhancement may appear as a characteristic spider leg appearance (see Image. Tuberculous Meningitis on Computed Tomography).[48] Imaging features are specific (95–100%) for TBM only when interpreted in combination. However, these findings have reduced sensitivity if taken individually.
TBM's basal pachymeningitis involves mostly the middle cerebral artery's M1 segment, circle of Willis, and lenticulostriate and thalamus-perforating arteries, causing a stroke.[49] The vasculitic infarcts are usually bilateral and multiple, mostly affecting the periventricular area, basal nuclei, and internal capsule. Magnetic resonance angiography helps delineate vascular narrowing in cerebral infarction territories.[50] Diffusion-weighted imaging is an advanced MRI technique that accurately identifies TBM-related cerebral vasculitis and varying stroke stages.[51]
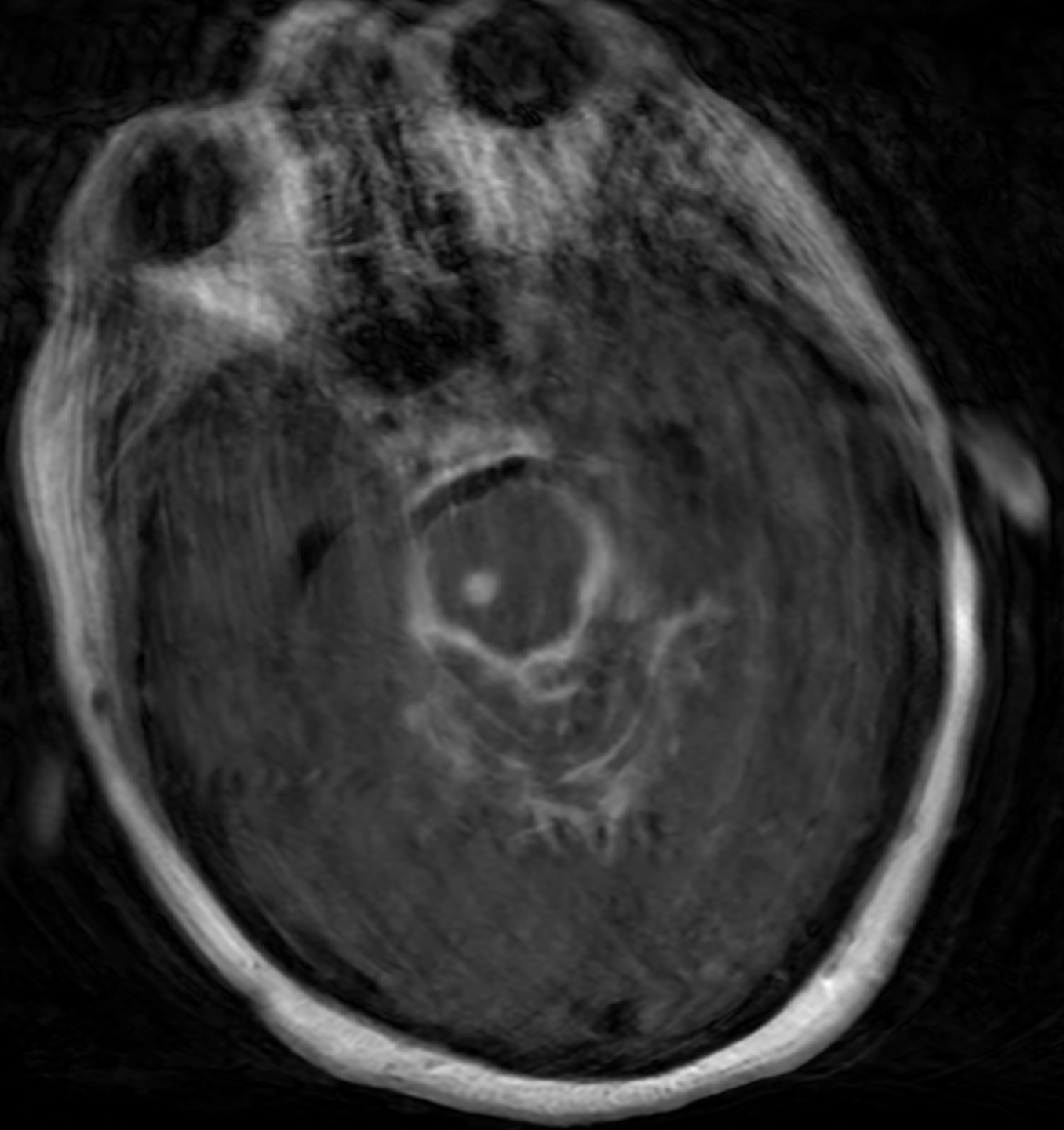
Tuberculomas are visible as discrete, single, or numerous ring-enhancing lesions with marked perilesional edema commonly located at the corticomedullary junction. (see Image. Brain Tuberculoma on MRI). Tuberculomas may appear differently on CT scans depending on their maturation stage, presenting as either noncaseating or solidly caseating lesions. CT may also demonstrate caseating masses with central liquefication or calcification (target sign).[52][53]
MRI often shows hypointensities on T1W and hyperintensities on T2W images. Postcontrast images delineate either ring-shaped or homogeneous disc-shaped enhancement (see Image. Brain Tuberculoma on Magnetic Resonance Imaging). Tuberculomas' unique metabolite pattern on magnetic resonance spectroscopy may differentiate them from metastases and gliomas. A complete and regular peripheral hypointense ring in susceptibility-weighted imaging (SWI) favors the diagnosis of tuberculomas.[54]
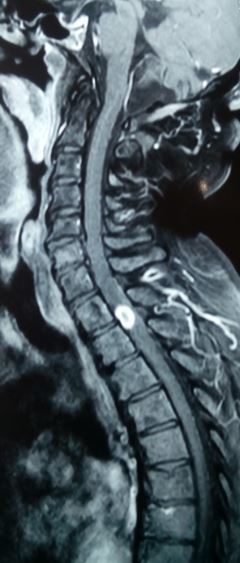
Tuberculous myelitis may be visible as diffuse cord swelling with an altered signal on MRI. Phase-contrast MRI is the technique of choice for identifying spinal subarachnoid space obliteration, dural thickening, paraspinal exudates, cauda equina nerve root clumps, CSF loculations, cord infarction, and syringomyelia in spinal arachnoiditis.[55][56]
Treatment / Management
Considering CNS-TB's high mortality and morbidity rates and various diagnostic challenges, empiric treatment with antituberculous therapy (ATT) and adjunctive corticosteroids may commence based on strong clinical suspicion. Patients must be closely monitored for prompt treatment of adverse drug reactions and disease-related complications. No randomized clinical trials can ascertain the most favorable drug combination, dosage, and duration for treating this condition. Various guidelines recommend different treatment regimens.[57][58][59][60] (A1)
Drug susceptibility data (when available) and clinical response must always be considered regardless of the combination chosen. The optimal treatment duration is uncertain.
Drug-Susceptible Central Nervous System Tuberculosis
The current guidelines recommend 4 orally administered drugs: isoniazid (INH) 5 mg/kg/d, rifampicin (RMP) 10 mg/kg/d, pyrazinamide (PZA) 25 mg/kg/d, and ethambutol (ETB) 15 mg/kg/d for the first 2 months, constituting the intensive phase. The continuation phase includes INH, RMP, and ETB daily for at least 7 months. PZA and EMB may be discontinued in the maintenance phase. PZA omission in the intensive phase warrants extending treatment to 18 months. Directly observed therapy short-course, which involves thrice-weekly drug administration, is currently not the standard of care for CNS-TB in India.
INH displays early bactericidal activity. RMP has excellent sterilizing activity, clearing persistent bacilli. PZA penetrates the BBB readily. ETB is a weak drug but completes the regimen for drug-susceptible tuberculosis. Drugs that may replace ETB include streptomycin, levofloxacin, and ethionamide. ETB is avoided when the patient's vision is compromised at baseline or cannot be assessed regularly. Streptomycin is contraindicated in pregnancy, kidney impairment, and hearing loss. Levofloxacin is superior to gatifloxacin or ciprofloxacin.[61] Higher RMP or levofloxacin doses are not associated with better survival.[62](A1)
OCA management continues to be a challenge. The response is usually disappointing, and pulse corticosteroids, thalidomide, and hyaluronidase have been tried with inconsistent outcomes. Neurosurgical intervention and its optimum timing are debatable. Paradoxical OCA also has a variable response.[63]
Drug-Resistant Central Nervous System Tuberculosis
The guidelines for managing drug-resistant CNS-TB are similar to those for PTB. The key principle involves never adding a single drug to a failing regimen. Management is challenging due to the huge number of drugs with overlapping adverse drug reaction profiles. The newer agents, bedaquiline and delamanid, received accelerated approvals for PTB but not CNS-TB.[64]
Tuberculomas
International guidelines recommend treating tuberculomas with standard ATT for CNS-TB for 9 to 12 months.[65][66] Clinical improvement and radiological clearance are observed in 90% and 80% of patients, respectively, by 9 months. Complete resolution depends upon the lesion's initial size. Tuberculomas smaller than 2.5 cm resolve in 5 to 8 months, while 50% of lesions larger than 2.5 cm take over 12 months to disappear.[67] Additionally, guidelines recommend that every patient should receive adjunctive steroids, especially in the presence of paradoxical responses.[68] Surgical intervention is indicated in patients unresponsive to medical management or with raised intracranial pressure.[65](B2)
Spinal Arachnoiditis
Spinal tuberculosis is managed similarly to other EPTB forms. Most international guidelines recommend daily therapy for 6 to 9 months, possibly extending to 24 months if the patient's response is unsatisfactory.[69] The conservative approach generally has functional outcomes similar to those of surgical treatment. Decompression surgery should be considered in individuals with progressive IRIS-unrelated neurological deficits.[70][71]
Anti-Inflammatory Drugs and Supportive Therapy
The use of adjunctive steroid therapy in TBM management remains an area of ongoing debate. A recent meta-analysis identified clinical benefits when dexamethasone was combined with standard ATT regimens for treating TBM.[72] Few robust, prospective randomized control studies provide definitive data on adjunctive steroid therapy's efficacy in this setting. Thwaites et al's randomized study found that adjunctive steroid therapy provided a survival benefit but did not prevent TBM-associated disability.[73](A1)
Donovan et al's recent, large randomized study found no survival benefit to using adjunctive dexamethasone in TBM treatment in patients with HIV.[74] Steroids are generally recommended for all TBM stages, except in stage 1 (mild disease), where the clinician may use their judgment. Dexamethasone's daily dose is 0.6 mg/kg/d in children and 0.4 mg/kg/d in adults. Prednisolone is an alternative used in doses of 2 to 4 mg/kg/d in children and 2.5 mg/kg/d in adults for a minimum of 4 weeks, followed by gradual tapering over the next 4 weeks.[75] Steroids have been shown to improve survival in TBM but probably do not prevent residual neurologic deficits and severe disability.[76] (A1)
Thalidomide's anti-inflammatory properties may be used to aid in recovering vision in OCA and IRIS cases.[77] Infliximab has also been shown to reduce brain inflammation in TBM with paradoxical reactions.[78] Aspirin has demonstrated a reduced 3-month stroke-related mortality rate in adults with TBM.[79] Neurocritical management of CNS-TB is also essential to interprofessional care.[80] Besides managing seizures and ischemic stroke, TBM-induced hyponatremia requires careful administration of oral salt, intravenous hypertonic saline, or, in resistant cases, fludrocortisone.[81][82](A1)
Immune Reconstitution Inflammatory Syndrome
Clinical or radiological deterioration may occur several weeks after ATT initiation in approximately one-third of patients with CNS-TB due to an aberrant immune response.[83] Significant IRIS predictors include HIV coinfection, younger age, female sex, and shorter illness duration.[84] TB-IRIS is suspected when patients who initially improve on ATT begin demonstrating worsening symptoms, tuberculomas with increasing size or number on imaging, or OCA aggravation. No test currently exists to confirm TB-IRIS.[85][86]
IRIS is believed to result from a delayed type-IV hypersensitivity reaction triggered by the significant release of mycobacterial antigens and increased tumor necrosis factor-α and CSF lymphocytes or protein levels. IRIS manifests with CN deficits due to nerve entanglement with inflammatory exudates. Conditions like communicating hydrocephalus, spinal arachnoiditis, and syringomyelia may also develop. However, the clinician's judgment is crucial in excluding other infections or potential treatment failure.
HIV Coinfection
Patients with HIV and TBM naive to antiretroviral therapy (ART) should begin ART 8 to 10 weeks after starting ATT. This sequence helps avert early TB-associated IRIS and its potentially fatal neurologic complications. ATT and ART coadministration in patients with HIV and CNS-TB is often complicated by numerous drug interactions and higher adverse drug reaction rates.[87]
Shunt Surgery in Tuberculous Meningitis
Communicating hydrocephalus is initially managed with corticosteroids, the carbonic anhydrase inhibitor acetazolamide, and osmotic diuretics like mannitol and glycerol. Temporary external ventricular drainage is also used for CSF drainage.[88] Endoscopic third ventriculostomy (ETV) has been reported to have a success rate of 73% to 89%.[89] ETV has fewer complications and may be more beneficial than ventriculoperitoneal shunt (VPS) placement. However, ETV is likely to fail during the illness' initial stages, in which case, VPS surgery may be performed.[90][91]. The outcome of patients treated with VPS placement depends considerably on the TBM clinical stage.[92] (A1)
Vellore and modified Vellore grading systems have been validated for patients with TBM with hydrocephalus to aid in surgical decision-making and predict the success of VPS surgery and ETV. The grades vary from 1 to 4, increasing in disease severity. The modified version incorporates the Glasgow Coma Scale score. The management of patients with grade 4 TBM is still contentious.[93]
Differential Diagnosis
TBM presents a significant diagnostic challenge due to its broad range of potential mimics. Meticulous exclusion of other causes of chronic meningitis or meningoencephalitis is crucial, especially in immunocompromised individuals. Conditions such as fungal meningitis (particularly cryptococcal meningitis), neurobrucellosis, neurosyphilis, neuroborreliosis, and neurosarcoidosis may mimic TBM. Partially treated bacterial meningitis, neoplastic meningitis, or systemic inflammation involving the CNS should also be considered in appropriate clinical settings. Neuroradiology and neuropathology are pivotal in aiding clinicians in these diagnostic challenges.
Tuberculoma has similar clinical features and radiological appearance as neurocysticercosis, cryptococcoma, CNS lymphoma, and primary or metastatic brain tumor. Focal encephalitides like herpes simplex and parameningeal infections like brain abscesses may occasionally behave similarly to CNS tuberculoma. CNS toxoplasmosis requires careful consideration in cases of HIV coinfection.
Spinal arachnoiditis needs to be differentiated from an intradural spinal tuberculoma, extradural compressive myelopathy due to tuberculous spondylitis with paravertebral cold abscess, noncompressive acute transverse myelitis, and Guillain-Barre syndrome. Cytomegalovirus polyradiculopathy can confuse the picture in people with HIV.
Pertinent Studies and Ongoing Trials
Developments in the fields of host immune responses, pathogenesis, and tuberculosis diagnosis and treatment are happening globally.[94] Emerging techniques for rapid TBM diagnosis include measuring new biomarkers like CSF lactate, mNGS, cell-free DNA analysis, clustered regularly interspaced short palindromic repeats (CRISPR) technology for detecting low MTB levels in CSF, and peripheral blood microRNA detection using transcriptomic techniques.[95] Human genetic polymorphisms that can explain the heterogeneity of host immune response to anti-inflammatory therapies are being identified. Genomic and metabolomic studies are determining survival-predicting CSF biomarkers (αβ T-cells, natural killer cells, and pro-resolving mediators) that may be used to tailor and individualize therapy.[96][97]
Advanced imaging techniques and sequences like 3-dimensional magnetization-prepared rapid gradient-echo are being studied to better delineate brain lesions. Phase-contrast MRI is another novel and sensitive technique that helps assess altered CSF flow dynamics in patients with TBM.[98]
Novel promising drugs such as linezolid, bedaquiline, and pretomanid are being tested in various combinations, especially in highly drug-resistant MTB therapy.[99] Clinical trials currently examine the effectiveness of high-dose RIF and fluoroquinolones. A combination of intravenous INH, high-dose RIF, and ETB are also in the pipeline.[100]
Aspirin is being investigated as an adjunctive treatment to reduce infarction incidence due to its ability to inhibit CSF thromboxane A2 and upregulate pre-resolvins.[101] Adjunctive anti-tumor necrosis factor-α, interferon-γ, and thalidomide have also been tried to modulate host immune response. Suitable strategies are likewise being designed and implemented to accomplish the End Tuberculosis Strategy's global targets.[102][103]
Toxicity and Adverse Effect Management
ATT is generally well tolerated. However, close follow-up is needed to monitor any adverse drug reactions that may arise due to the drugs' cumulative effects and long exposure duration.[104] All ATT drugs have specific side effects besides gastrointestinal intolerance and fatigue. Factors such as HIV coinfection, drug resistance, and steroid prescription can exacerbate the problem. INH, RMP, and PZA are all hepatotoxic. INH can also cause peripheral neuropathy, but this reaction may be prevented by pyridoxine supplementation. INH can cause lupus-like symptoms, pellagra, and psychosis in rare instances. RMP discolors the urine and may cause rashes, influenza-like illness, and thrombocytopenia.
ETB may produce dose-dependent optic neuritis and peripheral neuropathy. PZA may give rise to various cutaneous reactions, asymptomatic hyperuricemia, and gouty arthritis. Streptomycin is associated with ototoxicity, vertigo, and injection site pain. The novel drug bedaquiline is associated with QT prolongation on electrocardiography.
All patients on ATT should have liver monitoring during the initial 8 weeks of treatment. An asymptomatic rise in hepatic transaminases is expected at the beginning of therapy. Hepatotoxic drugs should be replaced with 2nd- or 3rd-line options if transaminase levels exceed 3 to 5 times the upper limit of normal or serum bilirubin increases beyond 2.5 mg/L.[105][106]
Staging
TBM is classified into clinical stages based on illness severity when treatment begins. These stages help prognosticate and decide on therapy.[107][108] The TBM stages are defined as follows:
- Stage I: The patient is alert and conscious with no focal neurological signs or hydrocephalus.
- Stage II: The patient is confused or irritable and has few focal signs, such as CN palsies or hemiparesis.
- Stage III: The patient is comatose with or without dense neurological deficit and may be in decerebrate or decorticate posturing.
Prognosis
CNS-TB's prognosis is dismal even after therapy. TBM's mortality ranges between 9.8% and 57%, as it is associated with distressing levels of neurologic sequelae that disproportionately afflict children.[109] Various mortality and neurological morbidity predictors have been identified, including age, symptom duration, Glasgow Coma Scale score, absence of headache, CSF protein level, arachnoiditis extent, presence of complications, and Medical Research Council staging at presentation.[110]
Complications
CNS-TB can be lethal or cause devastating complications. The initial complications arise from pachymeningitis, leading to hydrocephalus, OCA-related visual loss, focal neurological deficits, stroke, arteritis-induced hemiplegias, cerebral edema, tuberculoma- or abscess-induced seizures, and diabetes insipidus or syndrome of inappropriate antidiuretic hormone secretion. Myelitis complicates TBM in 10% of cases and is an independent predictor of poor outcomes.[111] Around 10% to 30% of patients with TBM develop long-term residual neurologic deficits, including the above complications, besides learning disabilities, dementia, endocrine syndromes, and gait disturbances.
Postoperative and Rehabilitation Care
TBM necessitates intensive rehabilitation and optimal medical treatment to reduce disability and facilitate recovery. Rehabilitation should commence during hospitalization or in the intensive care unit and extend into the home postdischarge. Rehabilitative efforts should encompass multimodal sensory stimulation to enhance brain function recovery, scheduled posturing and positioning to prevent immobilization complications, passive and active range of motion exercises to maintain muscle strength, and chest physiotherapy to mitigate pneumonia and aspiration risks. Long-term rehabilitation is essential for patients with lingering neurological impairments[112]
Consultations
Specialists in the following disciplines should be consulted for comprehensive CNS-TB management:
- Infectious disease
- Neuroradiology
- Neurology
- Neurosurgery
- Ophthalmology
- Physiotherapy
Deterrence and Patient Education
Patients and their caregivers must be educated about medication compliance, as CNS-TB requires prolonged treatment. These individuals must be enlightened about the potential disease- and treatment-related complications. Patients and their families should be counseled for regular hospital visits, investigations, and close follow-ups to ensure disease resolution and prevent adverse drug reactions.
Pearls and Other Issues
Pearls
CNS-TB poses a diagnostic and therapeutic challenge due to its paucibacillary nature and diverse clinical manifestations. Diagnosis hinges on a combination of clinical suspicion, imaging, CSF analysis, and molecular techniques like Xpert MTB/RIF. Early and accurate diagnosis is crucial, as delayed treatment may lead to serious morbidity and mortality. Treatment involves ATT and adjunctive corticosteroids, with surgical intervention reserved for specific complications. Monitoring for paradoxical reactions and IRIS is essential during treatment. Long-term neurorehabilitation may be necessary for patients with residual neurological deficits. Interprofessional collaboration is paramount for optimizing outcomes in CNS-TB management.
Other Issues
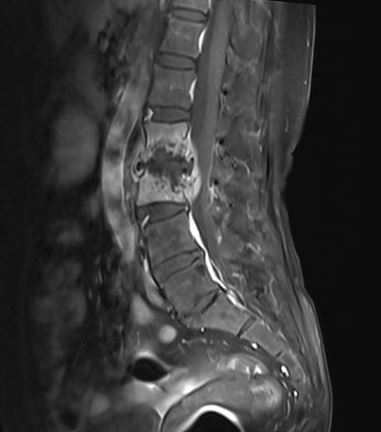
A discussion of spinal tuberculosis is relevant in this section due to its overlapping neurological presentations with CNS-TB. Spinal tuberculosis, a subset of skeletal tuberculosis, manifests as spondylodiscitis or spondylitis without intervertebral disk involvement.[113] In adults, intervertebral disk involvement often results from contiguous spread from infected vertebrae. In children, hematogenous spread may lead to TB diskitis due to the area's rich vascular supply. Pott disease is characterized by osteomyelitis and arthritis and typically affects multiple vertebrae. The condition produces progressive vertebral destruction, anterior collapse, kyphotic or gibbous deformity (in thoracic vertebrae), paraspinal cold abscess formation, and spinal canal narrowing. These complications can culminate in various neurological deficits, with paraplegia being the most devastating.[114]
Early-onset paraplegia may occur due to mechanical compression by tuberculous debris, bone or disk sequestrum, tuberculomas, granulomas, or abscess in extradural, intradural, or intramedullary regions, myelitis with cord edema, meningeal inflammation, and anterior spinal artery thrombosis with cord infarction. Frank infarction is rare since the smaller end-arteries are involved.[115]
Late-onset paraplegia in severe kyphosis may result from cord transection by a transverse bony ridge or dural fibrosis with cord encasement and, occasionally, syrinx formation. Paraspinal abscesses may mechanically compress the cord depending on their location. Retropharyngeal abscesses may cause dysphagia and respiratory distress. Mediastinal lesions can track down to the trachea, esophagus, or pleural cavity. Tuberculous abscesses may present as fusiform or bulbous paravertebral swelling in the thoracic region and groin or medial thigh bulging in the lumbar area.
For neuroimaging, spinal CT scans offer detailed bony information, while MRI is essential for evaluating soft tissue components (see Images. Spinal Cord Tuberculoma, Pott Spine). Spinal tuberculosis typically spares the intervertebral disc, shows vertebral endplate rarefaction and anterior vertebral wedging, and may present with paravertebral abscesses. Neuroimaging-guided needle biopsy of the involved vertebral body is the diagnostic gold standard. Pott spine treatment involves standard ATT, with the continuation phase potentially extending up to 18 months. Surgery for this condition is indicated in cases of neurological deficits, large paravertebral abscesses, and spinal instability.
Enhancing Healthcare Team Outcomes
CNS-TB, though rare, profoundly affects both the quantity and quality of life. Screening for active infected cases in high tuberculosis-burden settings may help prevent CNS-TB. Treatment adherence significantly influences outcomes once diagnosed. While most countries have national policies in place, embracing global evidence-based approaches to CNS-TB management is essential for ensuring optimal patient care and support.[116]
CNS-TB requires comprehensive and coordinated team care to enhance patient outcomes. Each team member, including the treating clinician, infectious disease specialist, neuroradiologist, neurologist, neurosurgeon, ophthalmologist, nurse, and pharmacist, holds individual and collaborative responsibilities. Nurses are crucial for overall patient care, while pharmacists, especially infectious disease-specialized pharmacists, are vital during drug selection, medication reconciliation, dosing regimens, adjustments, and patient counseling.[117][118] Effective communication with prescribing clinicians is essential for pharmacists to modify therapy appropriately in response to patient status changes.
Effective communication and collaboration within the healthcare team are essential for shared decision-making to reduce complications and expedite recovery in critically ill patients.[119] Improved infection control modalities and surveillance are necessary to screen and diagnose patients efficiently and provide interprofessional services to affected individuals.
Most countries release evidence-based guidelines for managing PTB and EPTB without considering each patient's unique circumstances, geographic location, and healthcare setting. Clinical decisions must be individualized to enhance health outcomes.[120]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
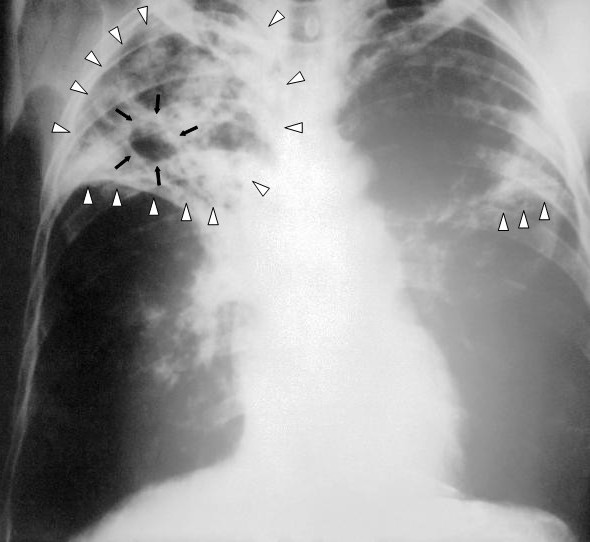
Pulmonary Tuberculosis. This image shows an anteroposterior chest x-ray of a patient diagnosed with advanced bilateral pulmonary tuberculosis. The x-ray reveals the presence of bilateral pulmonary infiltrate (white triangles), and "caving formation" (black arrows) present in the right apical region. The findings suggest far-advanced tuberculosis.
Centers for Disease Control and Prevention
References
Erdem H, Inan A, Guven E, Hargreaves S, Larsen L, Shehata G, Pernicova E, Khan E, Bastakova L, Namani S, Harxhi A, Roganovic T, Lakatos B, Uysal S, Sipahi OR, Crisan A, Miftode E, Stebel R, Jegorovic B, Fehér Z, Jekkel C, Pandak N, Moravveji A, Yilmaz H, Khalifa A, Musabak U, Yilmaz S, Jouhar A, Oztoprak N, Argemi X, Baldeyrou M, Bellaud G, Moroti RV, Hasbun R, Salazar L, Tekin R, Canestri A, Čalkić L, Praticò L, Yilmaz-Karadag F, Santos L, Pinto A, Kaptan F, Bossi P, Aron J, Duissenova A, Shopayeva G, Utaganov B, Grgic S, Ersoz G, Wu AKL, Lung KC, Bruzsa A, Radic LB, Kahraman H, Momen-Heravi M, Kulzhanova S, Rigo F, Konkayeva M, Smagulova Z, Tang T, Chan P, Ahmetagic S, Porobic-Jahic H, Moradi F, Kaya S, Cag Y, Bohr A, Artuk C, Celik I, Amsilli M, Gul HC, Cascio A, Lanzafame M, Nassar M. The burden and epidemiology of community-acquired central nervous system infections: a multinational study. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2017 Sep:36(9):1595-1611. doi: 10.1007/s10096-017-2973-0. Epub 2017 Apr 10 [PubMed PMID: 28397100]
Leonard JM. Central Nervous System Tuberculosis. Microbiology spectrum. 2017 Mar:5(2):. doi: 10.1128/microbiolspec.TNMI7-0044-2017. Epub [PubMed PMID: 28281443]
Ingole R, Garg RK, Malhotra HS, Jain A, Kumar N, Rizvi I, Garg R. Spectrum of central nervous system tuberculosis: An experience from a large tertiary care institution of India. The Indian journal of tuberculosis. 2019 Jan:66(1):49-57. doi: 10.1016/j.ijtb.2017.05.011. Epub 2017 Jun 16 [PubMed PMID: 30797283]
Schaller MA, Wicke F, Foerch C, Weidauer S. Central Nervous System Tuberculosis : Etiology, Clinical Manifestations and Neuroradiological Features. Clinical neuroradiology. 2019 Mar:29(1):3-18. doi: 10.1007/s00062-018-0726-9. Epub 2018 Sep 17 [PubMed PMID: 30225516]
Török ME. Tuberculous meningitis: advances in diagnosis and treatment. British medical bulletin. 2015 Mar:113(1):117-31. doi: 10.1093/bmb/ldv003. Epub 2015 Feb 18 [PubMed PMID: 25693576]
Level 3 (low-level) evidenceDavis AG, Rohlwink UK, Proust A, Figaji AA, Wilkinson RJ. The pathogenesis of tuberculous meningitis. Journal of leukocyte biology. 2019 Feb:105(2):267-280. doi: 10.1002/JLB.MR0318-102R. Epub 2019 Jan 15 [PubMed PMID: 30645042]
Guleria R, Kavitha. Central nervous system tuberculosis. The Indian journal of tuberculosis. 2014 Jul:61(3):195-9 [PubMed PMID: 25241567]
Imran D, Estiasari R, Maharani K, Sucipto, Lestari DC, Yunus RE, Yunihastuti E, Karyadi TH, Oei D, Timan IS, Wulandari D, Wahyuningsih R, Adawiyah R, Kurniawan A, Mulyadi R, Karuniawati A, Jaya UA, Safari D, van Laarhoven A, Alisjahbana B, Dian S, Chaidir L, Ganiem AR, Lastri DN, Aye Myint KS, van Crevel R. Presentation, etiology, and outcome of brain infections in an Indonesian hospital: A cohort study. Neurology. Clinical practice. 2018 Oct:8(5):379-388. doi: 10.1212/CPJ.0000000000000517. Epub [PubMed PMID: 30564491]
Patkar D, Narang J, Yanamandala R, Lawande M, Shah GV. Central nervous system tuberculosis: pathophysiology and imaging findings. Neuroimaging clinics of North America. 2012 Nov:22(4):677-705. doi: 10.1016/j.nic.2012.05.006. Epub 2012 Aug 11 [PubMed PMID: 23122262]
Level 2 (mid-level) evidenceChin JH. Tuberculous meningitis: A neglected tropical disease? Neurology. Clinical practice. 2019 Apr:9(2):152-154. doi: 10.1212/CPJ.0000000000000606. Epub [PubMed PMID: 31041130]
Deutsch-Feldman M, Pratt RH, Price SF, Tsang CA, Self JL. Tuberculosis - United States, 2020. MMWR. Morbidity and mortality weekly report. 2021 Mar 26:70(12):409-414. doi: 10.15585/mmwr.mm7012a1. Epub 2021 Mar 26 [PubMed PMID: 33764959]
Filardo TD, Feng PJ, Pratt RH, Price SF, Self JL. Tuberculosis - United States, 2021. MMWR. Morbidity and mortality weekly report. 2022 Mar 25:71(12):441-446. doi: 10.15585/mmwr.mm7112a1. Epub 2022 Mar 25 [PubMed PMID: 35324877]
Rieder HL, Snider DE Jr, Cauthen GM. Extrapulmonary tuberculosis in the United States. The American review of respiratory disease. 1990 Feb:141(2):347-51 [PubMed PMID: 2301852]
Moule MG, Cirillo JD. Mycobacterium tuberculosis Dissemination Plays a Critical Role in Pathogenesis. Frontiers in cellular and infection microbiology. 2020:10():65. doi: 10.3389/fcimb.2020.00065. Epub 2020 Feb 25 [PubMed PMID: 32161724]
Dastur DK, Manghani DK, Udani PM. Pathology and pathogenetic mechanisms in neurotuberculosis. Radiologic clinics of North America. 1995 Jul:33(4):733-52 [PubMed PMID: 7610242]
Level 3 (low-level) evidenceCherian A, Ajitha KC, Iype T, Divya KP. Neurotuberculosis: an update. Acta neurologica Belgica. 2021 Feb:121(1):11-21. doi: 10.1007/s13760-020-01575-0. Epub 2021 Jan 5 [PubMed PMID: 33400226]
Bhargava S, Gupta AK, Tandon PN. Tuberculous meningitis--a CT study. The British journal of radiology. 1982 Mar:55(651):189-96 [PubMed PMID: 7066619]
Kent SJ, Crowe SM, Yung A, Lucas CR, Mijch AM. Tuberculous meningitis: a 30-year review. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1993 Dec:17(6):987-94 [PubMed PMID: 8110957]
Thomas MD, Chopra JS, Walia BN. Tuberculous meningitis (T.B.M.)(a clinical study of 232 cases). The Journal of the Association of Physicians of India. 1977 Sep:25(9):633-9 [PubMed PMID: 612660]
Level 3 (low-level) evidenceSalvador GLO, Basso ACN, Barbieri PP, Leitao CA, Teixeira BCA, Neto AC. Central nervous system and spinal cord tuberculosis: Revisiting an important disease. Clinical imaging. 2021 Jan:69():158-168. doi: 10.1016/j.clinimag.2020.07.020. Epub 2020 Jul 29 [PubMed PMID: 32853843]
Kocen RS, Parsons M. Neurological complications of tuberculosis: some unusual manifestations. The Quarterly journal of medicine. 1970 Jan:39(153):17-30 [PubMed PMID: 5427330]
Berenguer J, Moreno S, Laguna F, Vicente T, Adrados M, Ortega A, González-LaHoz J, Bouza E. Tuberculous meningitis in patients infected with the human immunodeficiency virus. The New England journal of medicine. 1992 Mar 5:326(10):668-72 [PubMed PMID: 1346547]
Méchaï F, Bouchaud O. Tuberculous meningitis: Challenges in diagnosis and management. Revue neurologique. 2019 Sep-Oct:175(7-8):451-457. doi: 10.1016/j.neurol.2019.07.007. Epub 2019 Aug 2 [PubMed PMID: 31383464]
Wilkinson RJ, Rohlwink U, Misra UK, van Crevel R, Mai NTH, Dooley KE, Caws M, Figaji A, Savic R, Solomons R, Thwaites GE, Tuberculous Meningitis International Research Consortium. Tuberculous meningitis. Nature reviews. Neurology. 2017 Oct:13(10):581-598. doi: 10.1038/nrneurol.2017.120. Epub 2017 Sep 8 [PubMed PMID: 28884751]
Marais S, Thwaites G, Schoeman JF, Török ME, Misra UK, Prasad K, Donald PR, Wilkinson RJ, Marais BJ. Tuberculous meningitis: a uniform case definition for use in clinical research. The Lancet. Infectious diseases. 2010 Nov:10(11):803-12. doi: 10.1016/S1473-3099(10)70138-9. Epub 2010 Sep 6 [PubMed PMID: 20822958]
Level 3 (low-level) evidenceKim MC, Park KH, Lee SA, Kim SH. Validation of the Uniform Case Definition Criteria for Differentiating Tuberculous Meningitis, Viral Meningitis, and Bacterial Meningitis in Adults. Infection & chemotherapy. 2019 Jun:51(2):188-190. doi: 10.3947/ic.2019.51.2.188. Epub [PubMed PMID: 31270999]
Level 3 (low-level) evidenceMunakomi S, Grasso G, Chapagain R. Multi-spectral Pattern of Clinical Presentation and the Resultant Outcome in Central Nervous System Tuberculosis: A Single Center Study on the Ubiquitous Pathogen. Advances in experimental medicine and biology. 2020:1271():29-35. doi: 10.1007/5584_2019_466. Epub [PubMed PMID: 31994016]
Level 3 (low-level) evidenceVerma R, Sarkar S, Garg RK, Malhotra HS, Sharma PK, Saxena S. Ophthalmological manifestation in patients of tuberculous meningitis. QJM : monthly journal of the Association of Physicians. 2019 Jun 1:112(6):409-419. doi: 10.1093/qjmed/hcz037. Epub [PubMed PMID: 30722057]
Stadelman AM, Ssebambulidde K, Buller A, Tugume L, Yuquimpo K, Bakker CJ, Boulware DR, Bahr NC. Cerebrospinal fluid AFB smear in adults with tuberculous meningitis: A systematic review and diagnostic test accuracy meta-analysis. Tuberculosis (Edinburgh, Scotland). 2022 Jul:135():102230. doi: 10.1016/j.tube.2022.102230. Epub 2022 Jun 24 [PubMed PMID: 35779498]
Level 1 (high-level) evidenceKohli M, Schiller I, Dendukuri N, Dheda K, Denkinger CM, Schumacher SG, Steingart KR. Xpert(®) MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance. The Cochrane database of systematic reviews. 2018 Aug 27:8(8):CD012768. doi: 10.1002/14651858.CD012768.pub2. Epub 2018 Aug 27 [PubMed PMID: 30148542]
Level 1 (high-level) evidenceWakode P, Siddaiah N, Manjunath N, Bahubali VKH. GeneXpert: A Rapid and Supplementary Diagnostic Tool for Tuberculous Meningitis, Experience from Tertiary Neurocenter. Journal of neurosciences in rural practice. 2022 Apr:13(2):204-210. doi: 10.1055/s-0041-1742138. Epub 2022 Feb 18 [PubMed PMID: 35694081]
Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, Allen J, Tahirli R, Blakemore R, Rustomjee R, Milovic A, Jones M, O'Brien SM, Persing DH, Ruesch-Gerdes S, Gotuzzo E, Rodrigues C, Alland D, Perkins MD. Rapid molecular detection of tuberculosis and rifampin resistance. The New England journal of medicine. 2010 Sep 9:363(11):1005-15. doi: 10.1056/NEJMoa0907847. Epub 2010 Sep 1 [PubMed PMID: 20825313]
Bahr NC, Nuwagira E, Evans EE, Cresswell FV, Bystrom PV, Byamukama A, Bridge SC, Bangdiwala AS, Meya DB, Denkinger CM, Muzoora C, Boulware DR, ASTRO-CM Trial Team. Diagnostic accuracy of Xpert MTB/RIF Ultra for tuberculous meningitis in HIV-infected adults: a prospective cohort study. The Lancet. Infectious diseases. 2018 Jan:18(1):68-75. doi: 10.1016/S1473-3099(17)30474-7. Epub 2017 Sep 14 [PubMed PMID: 28919338]
Rajput R, Singh P, Sarin R, Sethi P, Sharma S. Diagnostic accuracy of loop-mediated isothermal amplification assay for extra-pulmonary tuberculosis in Indian population. Journal of microbiological methods. 2019 Mar:158():59-65. doi: 10.1016/j.mimet.2019.01.016. Epub 2019 Jan 28 [PubMed PMID: 30703448]
Quinn CM, Kagimu E, Okirworth M, Bangdiwala AS, Mugumya G, Ramachandran PS, Wilson MR, Meya DB, Cresswell FV, Bahr NC, Boulware DR. Fujifilm SILVAMP TB LAM Assay on Cerebrospinal Fluid for the Detection of Tuberculous Meningitis in Adults With Human Immunodeficiency Virus. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2021 Nov 2:73(9):e3428-e3434. doi: 10.1093/cid/ciaa1910. Epub [PubMed PMID: 33388751]
Siddiqi OK, Birbeck GL, Ghebremichael M, Mubanga E, Love S, Buback C, Kosloff B, Ayles H, Atadzhanov M, Dheda K, Koralnik IJ. Prospective Cohort Study on Performance of Cerebrospinal Fluid (CSF) Xpert MTB/RIF, CSF Lipoarabinomannan (LAM) Lateral Flow Assay (LFA), and Urine LAM LFA for Diagnosis of Tuberculous Meningitis in Zambia. Journal of clinical microbiology. 2019 Aug:57(8):. doi: 10.1128/JCM.00652-19. Epub 2019 Jul 26 [PubMed PMID: 31189584]
Level 2 (mid-level) evidenceWen A, Leng EL, Liu SM, Zhou YL, Cao WF, Yao DY, Hu F. Diagnostic Accuracy of Interferon-Gamma Release Assays for Tuberculous Meningitis: A Systematic Review and Meta-Analysis. Frontiers in cellular and infection microbiology. 2022:12():788692. doi: 10.3389/fcimb.2022.788692. Epub 2022 Apr 22 [PubMed PMID: 35531329]
Level 1 (high-level) evidenceShi F, Qiu X, Yu M, Huang Y. Tuberculosis-specific antigen stimulated and unstimulated interferon-γ for tuberculous meningitis diagnosis: A systematic review and meta-analysis. PloS one. 2022:17(8):e0273834. doi: 10.1371/journal.pone.0273834. Epub 2022 Aug 30 [PubMed PMID: 36040925]
Level 2 (mid-level) evidenceChen Y, Wang Y, Liu X, Li W, Fu H, Liu X, Zhang X, Zhou X, Yang B, Yao J, Ma X, Han L, Li H, Zheng L. Comparative diagnostic utility of metagenomic next-generation sequencing, GeneXpert, modified Ziehl-Neelsen staining, and culture using cerebrospinal fluid for tuberculous meningitis: A multi-center, retrospective study in China. Journal of clinical laboratory analysis. 2022 Apr:36(4):e24307. doi: 10.1002/jcla.24307. Epub 2022 Feb 24 [PubMed PMID: 35202495]
Level 2 (mid-level) evidenceLin BW, Hong JC, Jiang ZJ, Zhang WQ, Fan QC, Yao XP. Performance of metagenomic next-generation sequencing in cerebrospinal fluid for diagnosis of tuberculous meningitis. Journal of medical microbiology. 2024 Mar:73(3):. doi: 10.1099/jmm.0.001818. Epub [PubMed PMID: 38506717]
Sun Q, Sha W, Xiao HP, Tian Q, Zhu H. Evaluation of cerebrospinal fluid adenosine deaminase activity for the differential diagnosis of tuberculous and nontuberculous meningitis. The American journal of the medical sciences. 2012 Aug:344(2):116-21. doi: 10.1097/MAJ.0b013e318238fee3. Epub [PubMed PMID: 22104430]
Level 2 (mid-level) evidenceRana SV, Chacko F, Lal V, Arora SK, Parbhakar S, Sharma SK, Singh K. To compare CSF adenosine deaminase levels and CSF-PCR for tuberculous meningitis. Clinical neurology and neurosurgery. 2010 Jun:112(5):424-30. doi: 10.1016/j.clineuro.2010.02.012. Epub 2010 Mar 29 [PubMed PMID: 20347212]
Chaudhary V, Bano S, Garga UC. Central Nervous System Tuberculosis: An Imaging Perspective. Canadian Association of Radiologists journal = Journal l'Association canadienne des radiologistes. 2017 May:68(2):161-170. doi: 10.1016/j.carj.2016.10.007. Epub 2017 Mar 7 [PubMed PMID: 28283299]
Level 3 (low-level) evidenceGarg RK, Malhotra HS, Jain A. Neuroimaging in tuberculous meningitis. Neurology India. 2016 Mar-Apr:64(2):219-27. doi: 10.4103/0028-3886.177608. Epub [PubMed PMID: 26954796]
Andronikou S, Smith B, Hatherhill M, Douis H, Wilmshurst J. Definitive neuroradiological diagnostic features of tuberculous meningitis in children. Pediatric radiology. 2004 Nov:34(11):876-85 [PubMed PMID: 15378213]
Botha H, Ackerman C, Candy S, Carr JA, Griffith-Richards S, Bateman KJ. Reliability and diagnostic performance of CT imaging criteria in the diagnosis of tuberculous meningitis. PloS one. 2012:7(6):e38982. doi: 10.1371/journal.pone.0038982. Epub 2012 Jun 29 [PubMed PMID: 22768055]
Garg RK, Paliwal V, Malhotra HS. Tuberculous optochiasmatic arachnoiditis: a devastating form of tuberculous meningitis. Expert review of anti-infective therapy. 2011 Sep:9(9):719-29. doi: 10.1586/eri.11.93. Epub [PubMed PMID: 21905782]
Bernaerts A, Vanhoenacker FM, Parizel PM, Van Goethem JW, Van Altena R, Laridon A, De Roeck J, Coeman V, De Schepper AM. Tuberculosis of the central nervous system: overview of neuroradiological findings. European radiology. 2003 Aug:13(8):1876-90 [PubMed PMID: 12942288]
Level 3 (low-level) evidenceSoni N, Kumar S, Shimle A, Ora M, Bathla G, Mishra P. Cerebrovascular complications in tuberculous meningitis-A magnetic resonance imaging study in 90 patients from a tertiary care hospital. The neuroradiology journal. 2020 Feb:33(1):3-16. doi: 10.1177/1971400919881188. Epub 2019 Oct 7 [PubMed PMID: 31589101]
Kalita J, Misra UK, Nair PP. Predictors of stroke and its significance in the outcome of tuberculous meningitis. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2009 Jul-Aug:18(4):251-8. doi: 10.1016/j.jstrokecerebrovasdis.2008.11.007. Epub [PubMed PMID: 19560677]
Level 2 (mid-level) evidenceShukla R, Abbas A, Kumar P, Gupta RK, Jha S, Prasad KN. Evaluation of cerebral infarction in tuberculous meningitis by diffusion weighted imaging. The Journal of infection. 2008 Oct:57(4):298-306. doi: 10.1016/j.jinf.2008.07.012. Epub 2008 Aug 28 [PubMed PMID: 18760486]
Level 3 (low-level) evidenceWasay M, Kheleani BA, Moolani MK, Zaheer J, Pui M, Hasan S, Muzaffar S, Bakshi R, Sarawari AR. Brain CT and MRI findings in 100 consecutive patients with intracranial tuberculoma. Journal of neuroimaging : official journal of the American Society of Neuroimaging. 2003 Jul:13(3):240-7 [PubMed PMID: 12889171]
Bargalló J, Berenguer J, García-Barrionuevo J, Ubeda B, Bargalló N, Cardenal C, Mercader JM. The "target sign": is it a specific sign of CNS tuberculoma? Neuroradiology. 1996 Aug:38(6):547-50 [PubMed PMID: 8880716]
Parry AH, Wani AH, Shaheen FA, Wani AA, Feroz I, Ilyas M. Evaluation of intracranial tuberculomas using diffusion-weighted imaging (DWI), magnetic resonance spectroscopy (MRS) and susceptibility weighted imaging (SWI). The British journal of radiology. 2018 Nov:91(1091):20180342. doi: 10.1259/bjr.20180342. Epub 2018 Jul 23 [PubMed PMID: 29987985]
Garg RK, Malhotra HS, Gupta R. Spinal cord involvement in tuberculous meningitis. Spinal cord. 2015 Sep:53(9):649-57. doi: 10.1038/sc.2015.58. Epub 2015 Apr 21 [PubMed PMID: 25896347]
Gupta R, Garg RK, Jain A, Malhotra HS, Verma R, Sharma PK. Spinal cord and spinal nerve root involvement (myeloradiculopathy) in tuberculous meningitis. Medicine. 2015 Jan:94(3):e404. doi: 10.1097/MD.0000000000000404. Epub [PubMed PMID: 25621686]
Level 2 (mid-level) evidenceNahid P, Dorman SE, Alipanah N, Barry PM, Brozek JL, Cattamanchi A, Chaisson LH, Chaisson RE, Daley CL, Grzemska M, Higashi JM, Ho CS, Hopewell PC, Keshavjee SA, Lienhardt C, Menzies R, Merrifield C, Narita M, O'Brien R, Peloquin CA, Raftery A, Saukkonen J, Schaaf HS, Sotgiu G, Starke JR, Migliori GB, Vernon A. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016 Oct 1:63(7):e147-e195. doi: 10.1093/cid/ciw376. Epub 2016 Aug 10 [PubMed PMID: 27516382]
Level 1 (high-level) evidenceThwaites G, Fisher M, Hemingway C, Scott G, Solomon T, Innes J, British Infection Society. British Infection Society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. The Journal of infection. 2009 Sep:59(3):167-87. doi: 10.1016/j.jinf.2009.06.011. Epub 2009 Jul 4 [PubMed PMID: 19643501]
. WHO consolidated guidelines on tuberculosis: Module 4: Treatment - Drug-susceptible tuberculosis treatment. 2022:(): [PubMed PMID: 35727905]
Sharma SK, Ryan H, Khaparde S, Sachdeva KS, Singh AD, Mohan A, Sarin R, Paramasivan CN, Kumar P, Nischal N, Khatiwada S, Garner P, Tharyan P. Index-TB guidelines: Guidelines on extrapulmonary tuberculosis for India. The Indian journal of medical research. 2017 Apr:145(4):448-463. doi: 10.4103/ijmr.IJMR_1950_16. Epub [PubMed PMID: 28862176]
Thwaites GE, Bhavnani SM, Chau TT, Hammel JP, Török ME, Van Wart SA, Mai PP, Reynolds DK, Caws M, Dung NT, Hien TT, Kulawy R, Farrar J, Ambrose PG. Randomized pharmacokinetic and pharmacodynamic comparison of fluoroquinolones for tuberculous meningitis. Antimicrobial agents and chemotherapy. 2011 Jul:55(7):3244-53. doi: 10.1128/AAC.00064-11. Epub 2011 Apr 18 [PubMed PMID: 21502621]
Level 1 (high-level) evidenceHeemskerk AD, Bang ND, Mai NT, Chau TT, Phu NH, Loc PP, Chau NV, Hien TT, Dung NH, Lan NT, Lan NH, Lan NN, Phong le T, Vien NN, Hien NQ, Yen NT, Ha DT, Day JN, Caws M, Merson L, Thinh TT, Wolbers M, Thwaites GE, Farrar JJ. Intensified Antituberculosis Therapy in Adults with Tuberculous Meningitis. The New England journal of medicine. 2016 Jan 14:374(2):124-34. doi: 10.1056/NEJMoa1507062. Epub [PubMed PMID: 26760084]
Peloquin CA, Davies GR. The Treatment of Tuberculosis. Clinical pharmacology and therapeutics. 2021 Dec:110(6):1455-1466. doi: 10.1002/cpt.2261. Epub 2021 Jun 5 [PubMed PMID: 33837535]
Li Y, Sun F, Zhang W. Bedaquiline and delamanid in the treatment of multidrug-resistant tuberculosis: Promising but challenging. Drug development research. 2019 Feb:80(1):98-105. doi: 10.1002/ddr.21498. Epub 2018 Dec 11 [PubMed PMID: 30548290]
Dian S, Ganiem AR, van Laarhoven A. Central nervous system tuberculosis. Current opinion in neurology. 2021 Jun 1:34(3):396-402. doi: 10.1097/WCO.0000000000000920. Epub [PubMed PMID: 33661159]
Level 3 (low-level) evidenceMarais S, Van Toorn R, Chow FC, Manesh A, Siddiqi OK, Figaji A, Schoeman JF, Meintjes G, Tuberculous Meningitis International Research Consortium. Management of intracranial tuberculous mass lesions: how long should we treat for? Wellcome open research. 2019:4():158. doi: 10.12688/wellcomeopenres.15501.2. Epub 2019 Oct 31 [PubMed PMID: 32047859]
Level 2 (mid-level) evidenceKhatri GD, Krishnan V, Antil N, Saigal G. Magnetic resonance imaging spectrum of intracranial tubercular lesions: one disease, many faces. Polish journal of radiology. 2018:83():e524-e535. doi: 10.5114/pjr.2018.81408. Epub 2018 Dec 29 [PubMed PMID: 30800191]
Suárez I, Gruell H, Heyckendorf J, Fünger S, Lichtenstein T, Jung N, Lehmann C, Unnewehr M, Fätkenheuer G, Lange C, Rybniker J. Intensified adjunctive corticosteroid therapy for CNS tuberculomas. Infection. 2020 Apr:48(2):289-293. doi: 10.1007/s15010-019-01378-3. Epub 2020 Jan 3 [PubMed PMID: 31900872]
Pandita A, Madhuripan N, Pandita S, Hurtado RM. Challenges and controversies in the treatment of spinal tuberculosis. Journal of clinical tuberculosis and other mycobacterial diseases. 2020 May:19():100151. doi: 10.1016/j.jctube.2020.100151. Epub 2020 Feb 28 [PubMed PMID: 32154388]
Rathod TN, Sathe AH, Marathe NA. It's Never Too Late: Neurological Outcome of Delayed Decompression in Tuberculosis of Spine. Global spine journal. 2021 Jun:11(5):716-721. doi: 10.1177/2192568220922209. Epub 2020 May 19 [PubMed PMID: 32875909]
Yong LN, Ahmedy F, Yin KN, Engkasan JP. Functional Outcomes in Spinal Tuberculosis: A Review of the Literature. Asian spine journal. 2021 Jun:15(3):381-391. doi: 10.31616/asj.2020.0086. Epub 2020 Sep 22 [PubMed PMID: 32951405]
Wang W, Gao J, Liu J, Qi J, Zhang Q. Clinical Efficacy of Dexamethasone in the Treatment of Patients with Tuberculous Meningitis: A Meta-Analysis. Contrast media & molecular imaging. 2022:2022():2180374. doi: 10.1155/2022/2180374. Epub 2022 Mar 30 [PubMed PMID: 35418812]
Level 1 (high-level) evidenceThwaites GE, Nguyen DB, Nguyen HD, Hoang TQ, Do TT, Nguyen TC, Nguyen QH, Nguyen TT, Nguyen NH, Nguyen TN, Nguyen NL, Nguyen HD, Vu NT, Cao HH, Tran TH, Pham PM, Nguyen TD, Stepniewska K, White NJ, Tran TH, Farrar JJ. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. The New England journal of medicine. 2004 Oct 21:351(17):1741-51 [PubMed PMID: 15496623]
Level 1 (high-level) evidenceDonovan J, Bang ND, Imran D, Nghia HDT, Burhan E, Huong DTT, Hiep NTT, Ngoc LHB, Thanh DV, Thanh NT, Wardhani ALS, Maharani K, Gasmara CP, Hanh NHH, Oanh PKN, Estiasari R, Thu DDA, Kusumaningrum A, Dung LT, Giang DC, Ha DTM, Lan NH, Chau NVV, Nguyet NTM, Geskus RB, Thuong NTT, Kestelyn E, Hamers RL, Phu NH, Thwaites GE, ACT HIV Investigators. Adjunctive Dexamethasone for Tuberculous Meningitis in HIV-Positive Adults. The New England journal of medicine. 2023 Oct 12:389(15):1357-1367. doi: 10.1056/NEJMoa2216218. Epub [PubMed PMID: 37819954]
Gundamraj S, Hasbun R. The Use of Adjunctive Steroids in Central Nervous Infections. Frontiers in cellular and infection microbiology. 2020:10():592017. doi: 10.3389/fcimb.2020.592017. Epub 2020 Nov 23 [PubMed PMID: 33330135]
Prasad K, Singh MB, Ryan H. Corticosteroids for managing tuberculous meningitis. The Cochrane database of systematic reviews. 2016 Apr 28:4(4):CD002244. doi: 10.1002/14651858.CD002244.pub4. Epub 2016 Apr 28 [PubMed PMID: 27121755]
Level 1 (high-level) evidenceSchoeman JF, Andronikou S, Stefan DC, Freeman N, van Toorn R. Tuberculous meningitis-related optic neuritis: recovery of vision with thalidomide in 4 consecutive cases. Journal of child neurology. 2010 Jul:25(7):822-8. doi: 10.1177/0883073809350507. Epub 2010 Jun 2 [PubMed PMID: 20519667]
Level 3 (low-level) evidenceJorge JH, Graciela C, Pablo AP, Luis SH. A life-threatening central nervous system-tuberculosis inflammatory reaction nonresponsive to corticosteroids and successfully controlled by infliximab in a young patient with a variant of juvenile idiopathic arthritis. Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases. 2012 Jun:18(4):189-91. doi: 10.1097/RHU.0b013e318258b725. Epub [PubMed PMID: 22647865]
Level 3 (low-level) evidenceMisra UK, Kalita J, Nair PP. Role of aspirin in tuberculous meningitis: a randomized open label placebo controlled trial. Journal of the neurological sciences. 2010 Jun 15:293(1-2):12-7. doi: 10.1016/j.jns.2010.03.025. Epub 2010 Apr 24 [PubMed PMID: 20421121]
Level 1 (high-level) evidenceDonovan J, Figaji A, Imran D, Phu NH, Rohlwink U, Thwaites GE. The neurocritical care of tuberculous meningitis. The Lancet. Neurology. 2019 Aug:18(8):771-783. doi: 10.1016/S1474-4422(19)30154-1. Epub 2019 May 17 [PubMed PMID: 31109897]
Nagotkar L, Shanbag P, Dasarwar N. Cerebral salt wasting syndrome following neurosurgical intervention in tuberculous meningitis. Indian pediatrics. 2008 Jul:45(7):598-601 [PubMed PMID: 18695284]
Level 3 (low-level) evidenceMisra UK, Kalita J, Tuberculous Meningitis International Research Consortium. Mechanism, spectrum, consequences and management of hyponatremia in tuberculous meningitis. Wellcome open research. 2019:4():189. doi: 10.12688/wellcomeopenres.15502.2. Epub 2021 Mar 29 [PubMed PMID: 32734004]
Dian S, Hermawan R, van Laarhoven A, Immaculata S, Achmad TH, Ruslami R, Anwary F, Soetikno RD, Ganiem AR, van Crevel R. Brain MRI findings in relation to clinical characteristics and outcome of tuberculous meningitis. PloS one. 2020:15(11):e0241974. doi: 10.1371/journal.pone.0241974. Epub 2020 Nov 13 [PubMed PMID: 33186351]
Singh AK, Malhotra HS, Garg RK, Jain A, Kumar N, Kohli N, Verma R, Sharma PK. Paradoxical reaction in tuberculous meningitis: presentation, predictors and impact on prognosis. BMC infectious diseases. 2016 Jun 21:16():306. doi: 10.1186/s12879-016-1625-9. Epub 2016 Jun 21 [PubMed PMID: 27329253]
Quinn CM, Poplin V, Kasibante J, Yuquimpo K, Gakuru J, Cresswell FV, Bahr NC. Tuberculosis IRIS: Pathogenesis, Presentation, and Management across the Spectrum of Disease. Life (Basel, Switzerland). 2020 Oct 29:10(11):. doi: 10.3390/life10110262. Epub 2020 Oct 29 [PubMed PMID: 33138069]
Garg RK, Malhotra HS, Kumar N. Paradoxical reaction in HIV negative tuberculous meningitis. Journal of the neurological sciences. 2014 May 15:340(1-2):26-36. doi: 10.1016/j.jns.2014.03.025. Epub 2014 Mar 19 [PubMed PMID: 24680563]
Sharma SK, Mohan A, Kadhiravan T. HIV-TB co-infection: epidemiology, diagnosis & management. The Indian journal of medical research. 2005 Apr:121(4):550-67 [PubMed PMID: 15817963]
Rajshekhar V. Management of hydrocephalus in patients with tuberculous meningitis. Neurology India. 2009 Jul-Aug:57(4):368-74. doi: 10.4103/0028-3886.55572. Epub [PubMed PMID: 19770534]
Yadav YR, Parihar VS, Todorov M, Kher Y, Chaurasia ID, Pande S, Namdev H. Role of endoscopic third ventriculostomy in tuberculous meningitis with hydrocephalus. Asian journal of neurosurgery. 2016 Oct-Dec:11(4):325-329 [PubMed PMID: 27695532]
Suryaningtyas W, Ranuh IGMAR, Parenrengi MA. Shunt exposure as a ventriculoperitoneal shunt complication: A case series. International journal of surgery case reports. 2021 Feb:79():484-491. doi: 10.1016/j.ijscr.2021.01.084. Epub 2021 Jan 27 [PubMed PMID: 33757268]
Level 2 (mid-level) evidenceKumar A, Singh K, Sharma V. Surgery in hydrocephalus of tubercular origin: challenges and management. Acta neurochirurgica. 2013 May:155(5):869-73. doi: 10.1007/s00701-013-1658-4. Epub 2013 Mar 16 [PubMed PMID: 23504056]
Level 2 (mid-level) evidenceRizvi I, Garg RK, Malhotra HS, Kumar N, Sharma E, Srivastava C, Uniyal R. Ventriculo-peritoneal shunt surgery for tuberculous meningitis: A systematic review. Journal of the neurological sciences. 2017 Apr 15:375():255-263. doi: 10.1016/j.jns.2017.02.008. Epub 2017 Feb 4 [PubMed PMID: 28320142]
Level 1 (high-level) evidenceRajshekhar V. Three Decades of Vellore Grading for Tuberculous Meningitis with Hydrocephalus: A Reappraisal. Neurology India. 2021 Nov-Dec:69(Supplement):S569-S574. doi: 10.4103/0028-3886.332251. Epub [PubMed PMID: 35103015]
Arshad A, Dayal S, Gadhe R, Mawley A, Shin K, Tellez D, Phan P, Venketaraman V. Analysis of Tuberculosis Meningitis Pathogenesis, Diagnosis, and Treatment. Journal of clinical medicine. 2020 Sep 14:9(9):. doi: 10.3390/jcm9092962. Epub 2020 Sep 14 [PubMed PMID: 32937808]
Ssebambulidde K, Gakuru J, Ellis J, Cresswell FV, Bahr NC. Improving Technology to Diagnose Tuberculous Meningitis: Are We There Yet? Frontiers in neurology. 2022:13():892224. doi: 10.3389/fneur.2022.892224. Epub 2022 May 30 [PubMed PMID: 35711276]
van Laarhoven A, Dian S, van Dorp S, Purnama F, Koeken VACM, Diandini E, Utami F, Livia R, Apriani L, Ardiansyah E, Ter Horst R, Netea MG, Achmad TH, Hill PC, Ruslami R, Alisjahbana B, Ussher JE, Indrati A, Verrall A, Ganiem AR, van Crevel R. Immune cell characteristics and cytokine responses in adult HIV-negative tuberculous meningitis: an observational cohort study. Scientific reports. 2019 Jan 29:9(1):884. doi: 10.1038/s41598-018-36696-3. Epub 2019 Jan 29 [PubMed PMID: 30696839]
Colas RA, Nhat LTH, Thuong NTT, Gómez EA, Ly L, Thanh HH, Mai NTH, Phu NH, Thwaites GE, Dalli J. Proresolving mediator profiles in cerebrospinal fluid are linked with disease severity and outcome in adults with tuberculous meningitis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2019 Nov:33(11):13028-13039. doi: 10.1096/fj.201901590R. Epub 2019 Oct 5 [PubMed PMID: 31500466]
Ashta A, Prakash A, Dixit R, Kumar N. Cerebrospinal Fluid Flow Analysis in Tuberculous Meningitis Using Phase Contrast Technique on 3 Tesla MRI: A New Paradigm and Our Initial Experience. Neurology India. 2022 May-Jun:70(3):1025-1031. doi: 10.4103/0028-3886.349627. Epub [PubMed PMID: 35864634]
Conradie F, Diacon AH, Ngubane N, Howell P, Everitt D, Crook AM, Mendel CM, Egizi E, Moreira J, Timm J, McHugh TD, Wills GH, Bateson A, Hunt R, Van Niekerk C, Li M, Olugbosi M, Spigelman M, Nix-TB Trial Team. Treatment of Highly Drug-Resistant Pulmonary Tuberculosis. The New England journal of medicine. 2020 Mar 5:382(10):893-902. doi: 10.1056/NEJMoa1901814. Epub [PubMed PMID: 32130813]
Saylor D. Neurologic Complications of Tuberculosis. Continuum (Minneapolis, Minn.). 2021 Aug 1:27(4):992-1017. doi: 10.1212/CON.0000000000001005. Epub [PubMed PMID: 34623101]
Mai NTH, Dobbs N, Phu NH, Colas RA, Thao LTP, Thuong NTT, Nghia HDT, Hanh NHH, Hang NT, Heemskerk AD, Day JN, Ly L, Thu DDA, Merson L, Kestelyn E, Wolbers M, Geskus R, Summers D, Chau NVV, Dalli J, Thwaites GE. A randomised double blind placebo controlled phase 2 trial of adjunctive aspirin for tuberculous meningitis in HIV-uninfected adults. eLife. 2018 Feb 27:7():. doi: 10.7554/eLife.33478. Epub 2018 Feb 27 [PubMed PMID: 29482717]
Level 1 (high-level) evidencePan Z, Zhang J, Bu Q, He H, Bai L, Yang J, Liu Q, Lyu J. The Gap Between Global Tuberculosis Incidence and the First Milestone of the WHO End Tuberculosis Strategy: An Analysis Based on the Global Burden of Disease 2017 Database. Infection and drug resistance. 2020:13():1281-1286. doi: 10.2147/IDR.S248875. Epub 2020 May 4 [PubMed PMID: 32440164]
Chakaya J, Petersen E, Nantanda R, Mungai BN, Migliori GB, Amanullah F, Lungu P, Ntoumi F, Kumarasamy N, Maeurer M, Zumla A. The WHO Global Tuberculosis 2021 Report - not so good news and turning the tide back to End TB. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2022 Nov:124 Suppl 1():S26-S29. doi: 10.1016/j.ijid.2022.03.011. Epub 2022 Mar 20 [PubMed PMID: 35321845]
Forget EJ, Menzies D. Adverse reactions to first-line antituberculosis drugs. Expert opinion on drug safety. 2006 Mar:5(2):231-49 [PubMed PMID: 16503745]
Level 3 (low-level) evidenceDhiman RK, Saraswat VA, Rajekar H, Reddy C, Chawla YK. A guide to the management of tuberculosis in patients with chronic liver disease. Journal of clinical and experimental hepatology. 2012 Sep:2(3):260-70. doi: 10.1016/j.jceh.2012.07.007. Epub 2012 Sep 21 [PubMed PMID: 25755442]
Abbara A, Chitty S, Roe JK, Ghani R, Collin SM, Ritchie A, Kon OM, Dzvova J, Davidson H, Edwards TE, Hateley C, Routledge M, Buckley J, Davidson RN, John L. Drug-induced liver injury from antituberculous treatment: a retrospective study from a large TB centre in the UK. BMC infectious diseases. 2017 Mar 24:17(1):231. doi: 10.1186/s12879-017-2330-z. Epub 2017 Mar 24 [PubMed PMID: 28340562]
Level 2 (mid-level) evidenceKennedy DH, Fallon RJ. Tuberculous meningitis. JAMA. 1979 Jan 19:241(3):264-8 [PubMed PMID: 102806]
Rock RB, Olin M, Baker CA, Molitor TW, Peterson PK. Central nervous system tuberculosis: pathogenesis and clinical aspects. Clinical microbiology reviews. 2008 Apr:21(2):243-61, table of contents. doi: 10.1128/CMR.00042-07. Epub [PubMed PMID: 18400795]
Level 3 (low-level) evidenceWang MG, Luo L, Zhang Y, Liu X, Liu L, He JQ. Treatment outcomes of tuberculous meningitis in adults: a systematic review and meta-analysis. BMC pulmonary medicine. 2019 Nov 6:19(1):200. doi: 10.1186/s12890-019-0966-8. Epub 2019 Nov 6 [PubMed PMID: 31694599]
Level 1 (high-level) evidenceGeorge EL, Iype T, Cherian A, Chandy S, Kumar A, Balakrishnan A, Vijayakumar K. Predictors of mortality in patients with meningeal tuberculosis. Neurology India. 2012 Jan-Feb:60(1):18-22. doi: 10.4103/0028-3886.93583. Epub [PubMed PMID: 22406774]
Level 2 (mid-level) evidenceJiang Y, Xu X, Guo Z, Liu Y, Lin J, Suo L, Jiang Y, Liu B, Lu T. Myelitis: A Common Complication of Tuberculous Meningitis Predicting Poor Outcome. Frontiers in neurology. 2022:13():830029. doi: 10.3389/fneur.2022.830029. Epub 2022 Mar 16 [PubMed PMID: 35370906]
Quinn CM, Kasibante J, Namudde A, Bangdiwala AS, Kabahubya M, Nakasujja N, Lofgren S, Elliott A, Boulware DR, Meya DB, Cresswell FV. Neurocognitive outcomes of tuberculous meningitis in a primarily HIV-positive Ugandan cohort. Wellcome open research. 2021:6():208. doi: 10.12688/wellcomeopenres.16967.2. Epub 2022 Mar 3 [PubMed PMID: 35949653]
Rasouli MR, Mirkoohi M, Vaccaro AR, Yarandi KK, Rahimi-Movaghar V. Spinal tuberculosis: diagnosis and management. Asian spine journal. 2012 Dec:6(4):294-308. doi: 10.4184/asj.2012.6.4.294. Epub 2012 Dec 14 [PubMed PMID: 23275816]
Garg RK, Somvanshi DS. Spinal tuberculosis: a review. The journal of spinal cord medicine. 2011:34(5):440-54. doi: 10.1179/2045772311Y.0000000023. Epub [PubMed PMID: 22118251]
Koplay M, Erdogan H, Sivri M, Nayman A, Paksoy Y. Unusual reason of spinal cord infarction: tuberculous meningitis. Acta neurologica Belgica. 2016 Mar:116(1):87-9. doi: 10.1007/s13760-015-0507-z. Epub 2015 Jul 5 [PubMed PMID: 26143304]
Cocozza AM, Linh NN, Nathavitharana RR, Ahmad U, Jaramillo E, Gargioni GEM, Fox GJ. An assessment of current tuberculosis patient care and support policies in high-burden countries. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2020 Jan 1:24(1):36-42. doi: 10.5588/ijtld.19.0183. Epub [PubMed PMID: 32005305]
Daftary A, Mitchell EMH, Reid MJA, Fekadu E, Goosby E. To End TB, First-Ever High-Level Meeting on Tuberculosis Must Address Stigma. The American journal of tropical medicine and hygiene. 2018 Nov:99(5):1114-1116. doi: 10.4269/ajtmh.18-0591. Epub [PubMed PMID: 30226149]
Tang ZQ, Jiang RH, Xu HB. Effectiveness of pharmaceutical care on treatment outcomes for patients with first-time pulmonary tuberculosis in China. Journal of clinical pharmacy and therapeutics. 2018 Dec:43(6):888-894. doi: 10.1111/jcpt.12746. Epub 2018 Jul 12 [PubMed PMID: 30003561]
Muthu V, Agarwal R, Dhooria S, Aggarwal AN, Behera D, Sehgal IS. Outcome of Critically Ill Subjects With Tuberculosis: Systematic Review and Meta-Analysis. Respiratory care. 2018 Dec:63(12):1541-1554. doi: 10.4187/respcare.06190. Epub 2018 Sep 11 [PubMed PMID: 30206126]
Level 1 (high-level) evidenceLewinsohn DM, Leonard MK, LoBue PA, Cohn DL, Daley CL, Desmond E, Keane J, Lewinsohn DA, Loeffler AM, Mazurek GH, O'Brien RJ, Pai M, Richeldi L, Salfinger M, Shinnick TM, Sterling TR, Warshauer DM, Woods GL. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017 Jan 15:64(2):111-115. doi: 10.1093/cid/ciw778. Epub [PubMed PMID: 28052967]
Level 1 (high-level) evidence