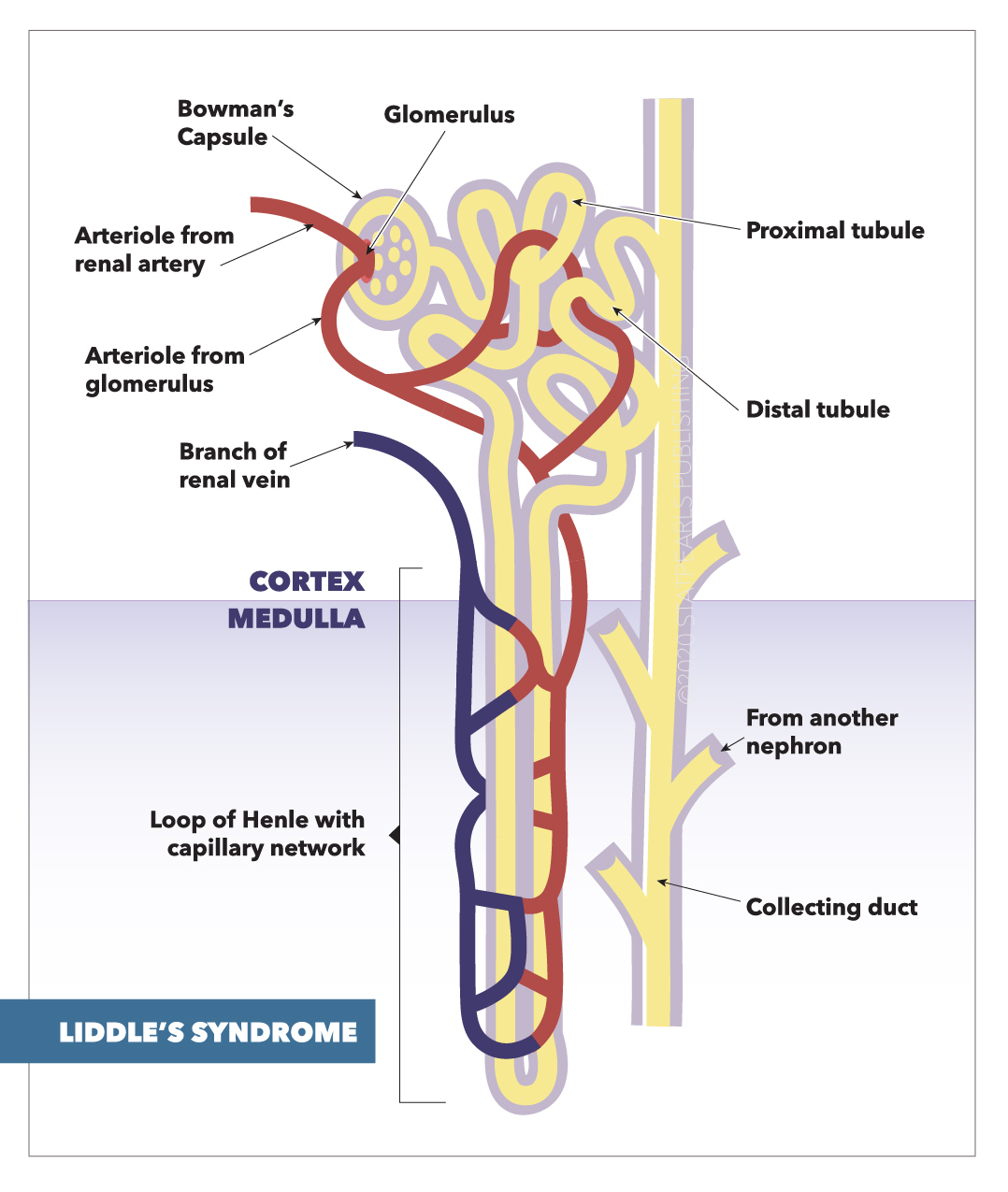
[1]
Wang LP, Yang KQ, Jiang XJ, Wu HY, Zhang HM, Zou YB, Song L, Bian J, Hui RT, Liu YX, Zhou XL. Prevalence of Liddle Syndrome Among Young Hypertension Patients of Undetermined Cause in a Chinese Population. Journal of clinical hypertension (Greenwich, Conn.). 2015 Nov:17(11):902-7. doi: 10.1111/jch.12598. Epub 2015 Jun 15
[PubMed PMID: 26075967]
[2]
Hansson JH, Nelson-Williams C, Suzuki H, Schild L, Shimkets R, Lu Y, Canessa C, Iwasaki T, Rossier B, Lifton RP. Hypertension caused by a truncated epithelial sodium channel gamma subunit: genetic heterogeneity of Liddle syndrome. Nature genetics. 1995 Sep:11(1):76-82
[PubMed PMID: 7550319]
[3]
Hanukoglu I, Hanukoglu A. Epithelial sodium channel (ENaC) family: Phylogeny, structure-function, tissue distribution, and associated inherited diseases. Gene. 2016 Apr 1:579(2):95-132. doi: 10.1016/j.gene.2015.12.061. Epub 2016 Jan 7
[PubMed PMID: 26772908]
[4]
Tetti M, Monticone S, Burrello J, Matarazzo P, Veglio F, Pasini B, Jeunemaitre X, Mulatero P. Liddle Syndrome: Review of the Literature and Description of a New Case. International journal of molecular sciences. 2018 Mar 11:19(3):. doi: 10.3390/ijms19030812. Epub 2018 Mar 11
[PubMed PMID: 29534496]
Level 3 (low-level) evidence
[5]
Liu K, Qin F, Sun X, Zhang Y, Wang J, Wu Y, Ma W, Wang W, Wu X, Qin Y, Zhang H, Zhou X, Wu H, Hui R, Zou Y, Jiang X, Song L. Analysis of the genes involved in Mendelian forms of low-renin hypertension in Chinese early-onset hypertensive patients. Journal of hypertension. 2018 Mar:36(3):502-509. doi: 10.1097/HJH.0000000000001556. Epub
[PubMed PMID: 28915228]
Level 2 (mid-level) evidence
[6]
Pagani L, Diekmann Y, Sazzini M, De Fanti S, Rondinelli M, Farnetti E, Casali B, Caretto A, Novara F, Zuffardi O, Garagnani P, Mantero F, Thomas MG, Luiselli D, Rossi E. Three Reportedly Unrelated Families With Liddle Syndrome Inherited From a Common Ancestor. Hypertension (Dallas, Tex. : 1979). 2018 Feb:71(2):273-279. doi: 10.1161/HYPERTENSIONAHA.117.10491. Epub 2017 Dec 11
[PubMed PMID: 29229744]
[7]
Tapolyai M, Uysal A, Dossabhoy NR, Zsom L, Szarvas T, Lengvárszky Z, Fülöp T. High prevalence of liddle syndrome phenotype among hypertensive US Veterans in Northwest Louisiana. Journal of clinical hypertension (Greenwich, Conn.). 2010 Nov:12(11):856-60. doi: 10.1111/j.1751-7176.2010.00359.x. Epub 2010 Aug 20
[PubMed PMID: 21054772]
[8]
Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994 Feb 3:367(6462):463-7
[PubMed PMID: 8107805]
[9]
Lu C, Pribanic S, Debonneville A, Jiang C, Rotin D. The PY motif of ENaC, mutated in Liddle syndrome, regulates channel internalization, sorting and mobilization from subapical pool. Traffic (Copenhagen, Denmark). 2007 Sep:8(9):1246-64
[PubMed PMID: 17605762]
[10]
Kellenberger S, Gautschi I, Rossier BC, Schild L. Mutations causing Liddle syndrome reduce sodium-dependent downregulation of the epithelial sodium channel in the Xenopus oocyte expression system. The Journal of clinical investigation. 1998 Jun 15:101(12):2741-50
[PubMed PMID: 9637708]
[11]
Nakada T, Koike H, Akiya T, Katayama T, Kawamata S, Takaya K, Shigematsu H. Liddle's syndrome, an uncommon form of hyporeninemic hypoaldosteronism: functional and histopathological studies. The Journal of urology. 1987 Apr:137(4):636-40
[PubMed PMID: 3550146]
[12]
Bubien JK. Epithelial Na+ channel (ENaC), hormones, and hypertension. The Journal of biological chemistry. 2010 Jul 30:285(31):23527-31. doi: 10.1074/jbc.R109.025049. Epub 2010 May 11
[PubMed PMID: 20460373]
[13]
Unwin RJ, Luft FC, Shirley DG. Pathophysiology and management of hypokalemia: a clinical perspective. Nature reviews. Nephrology. 2011 Feb:7(2):75-84. doi: 10.1038/nrneph.2010.175. Epub
[PubMed PMID: 21278718]
Level 3 (low-level) evidence
[14]
Amlal H, Krane CM, Chen Q, Soleimani M. Early polyuria and urinary concentrating defect in potassium deprivation. American journal of physiology. Renal physiology. 2000 Oct:279(4):F655-63
[PubMed PMID: 10997915]
[15]
Monticone S, Buffolo F, Tetti M, Veglio F, Pasini B, Mulatero P. GENETICS IN ENDOCRINOLOGY: The expanding genetic horizon of primary aldosteronism. European journal of endocrinology. 2018 Mar:178(3):R101-R111. doi: 10.1530/EJE-17-0946. Epub 2018 Jan 18
[PubMed PMID: 29348113]
[16]
Mulatero P, Verhovez A, Morello F, Veglio F. Diagnosis and treatment of low-renin hypertension. Clinical endocrinology. 2007 Sep:67(3):324-34
[PubMed PMID: 17573898]
[17]
Yang KQ, Lu CX, Fan P, Zhang Y, Meng X, Dong XQ, Luo F, Liu YX, Zhang HM, Wu HY, Cai J, Zhang X, Zhou XL. Genetic screening of SCNN1B and SCNN1G genes in early-onset hypertensive patients helps to identify Liddle syndrome. Clinical and experimental hypertension (New York, N.Y. : 1993). 2018:40(2):107-111. doi: 10.1080/10641963.2017.1334799. Epub 2017 Jul 18
[PubMed PMID: 28718682]
[18]
Warnock DG. Liddle syndrome: an autosomal dominant form of human hypertension. Kidney international. 1998 Jan:53(1):18-24
[PubMed PMID: 9452995]
[19]
Awadalla M, Patwardhan M, Alsamsam A, Imran N. Management of Liddle Syndrome in Pregnancy: A Case Report and Literature Review. Case reports in obstetrics and gynecology. 2017:2017():6279460. doi: 10.1155/2017/6279460. Epub 2017 Mar 15
[PubMed PMID: 28396810]
Level 3 (low-level) evidence
[20]
Wilson RC, Nimkarn S, New MI. Apparent mineralocorticoid excess. Trends in endocrinology and metabolism: TEM. 2001 Apr:12(3):104-11
[PubMed PMID: 11306334]
[21]
Kahle KT, Wilson FH, Lifton RP. Regulation of diverse ion transport pathways by WNK4 kinase: a novel molecular switch. Trends in endocrinology and metabolism: TEM. 2005 Apr:16(3):98-103
[PubMed PMID: 15808806]