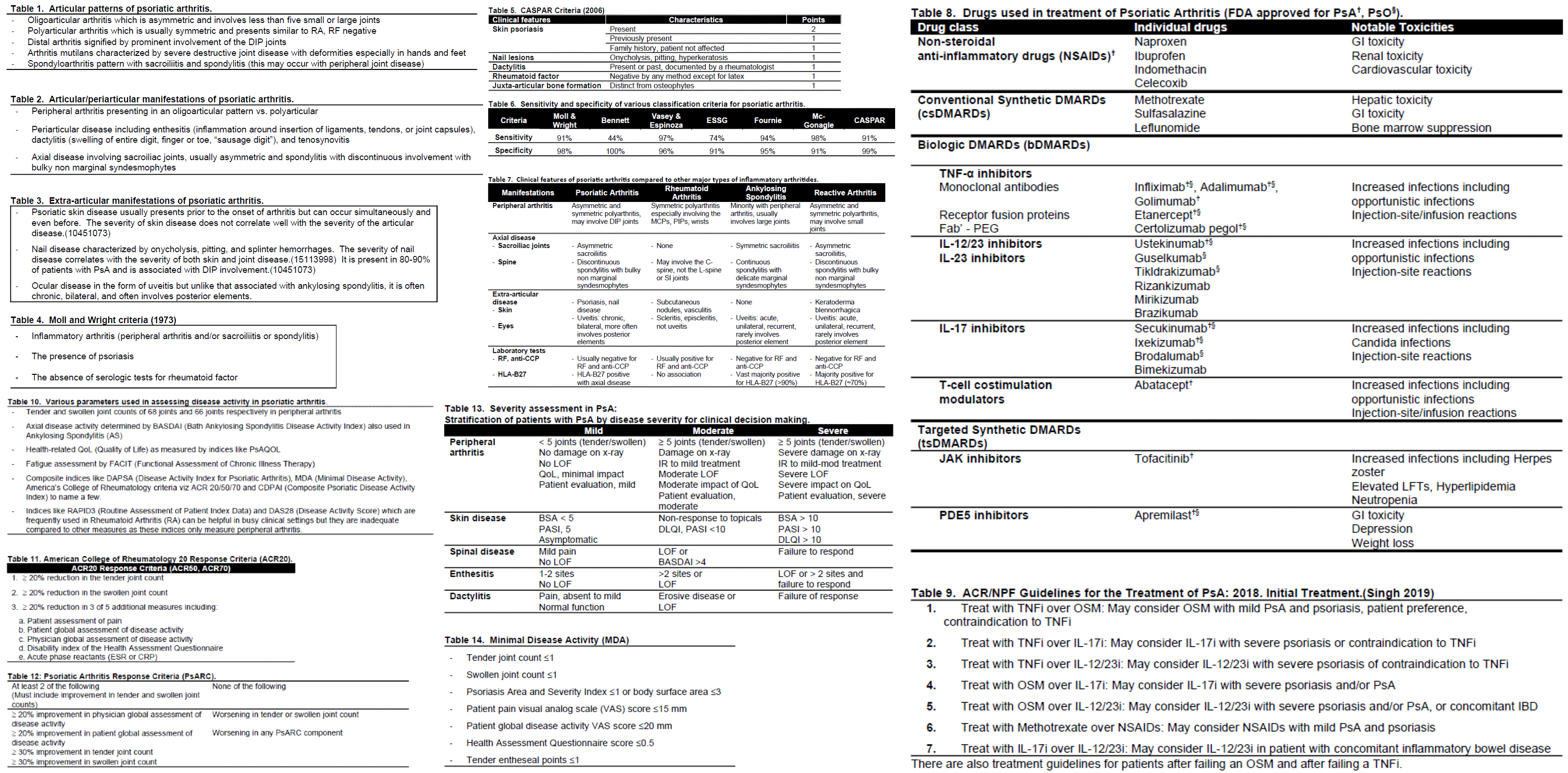
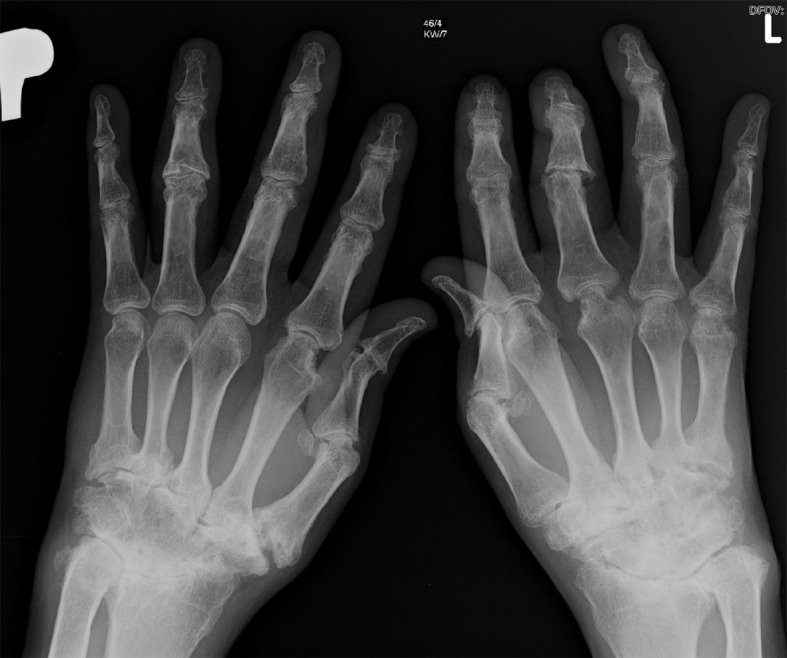
[1]
Karmacharya P, Chakradhar R, Ogdie A. The epidemiology of psoriatic arthritis: A literature review. Best practice & research. Clinical rheumatology. 2021 Jun:35(2):101692. doi: 10.1016/j.berh.2021.101692. Epub 2021 May 18
[PubMed PMID: 34016528]
[2]
Eder L, Haddad A, Rosen CF, Lee KA, Chandran V, Cook R, Gladman DD. The Incidence and Risk Factors for Psoriatic Arthritis in Patients With Psoriasis: A Prospective Cohort Study. Arthritis & rheumatology (Hoboken, N.J.). 2016 Apr:68(4):915-23. doi: 10.1002/art.39494. Epub
[PubMed PMID: 26555117]
[3]
FitzGerald O, Haroon M, Giles JT, Winchester R. Concepts of pathogenesis in psoriatic arthritis: genotype determines clinical phenotype. Arthritis research & therapy. 2015 May 7:17(1):115. doi: 10.1186/s13075-015-0640-3. Epub 2015 May 7
[PubMed PMID: 25948071]
[4]
Reveille JD. The genetic basis of spondyloarthritis. Annals of the rheumatic diseases. 2011 Mar:70 Suppl 1():i44-50. doi: 10.1136/ard.2010.140574. Epub
[PubMed PMID: 21339218]
[5]
Gladman DD, Anhorn KA, Schachter RK, Mervart H. HLA antigens in psoriatic arthritis. The Journal of rheumatology. 1986 Jun:13(3):586-92
[PubMed PMID: 3735281]
[6]
O'Rielly DD, Rahman P. Clinical and molecular significance of genetic loci associated with psoriatic arthritis. Best practice & research. Clinical rheumatology. 2021 Jun:35(2):101691. doi: 10.1016/j.berh.2021.101691. Epub 2021 May 19
[PubMed PMID: 34020887]
[7]
Nair RP, Stuart PE, Nistor I, Hiremagalore R, Chia NVC, Jenisch S, Weichenthal M, Abecasis GR, Lim HW, Christophers E, Voorhees JJ, Elder JT. Sequence and haplotype analysis supports HLA-C as the psoriasis susceptibility 1 gene. American journal of human genetics. 2006 May:78(5):827-851. doi: 10.1086/503821. Epub 2006 Mar 31
[PubMed PMID: 16642438]
[8]
Cafaro G, McInnes IB. Psoriatic arthritis: tissue-directed inflammation? Clinical rheumatology. 2018 Apr:37(4):859-868. doi: 10.1007/s10067-018-4012-7. Epub 2018 Feb 23
[PubMed PMID: 29476352]
[9]
Feld J, Chandran V, Haroon N, Inman R, Gladman D. Axial disease in psoriatic arthritis and ankylosing spondylitis: a critical comparison. Nature reviews. Rheumatology. 2018 Jun:14(6):363-371. doi: 10.1038/s41584-018-0006-8. Epub
[PubMed PMID: 29752461]
[10]
Feld J, Ye JY, Chandran V, Inman RD, Haroon N, Cook R, Gladman DD. Is axial psoriatic arthritis distinct from ankylosing spondylitis with and without concomitant psoriasis? Rheumatology (Oxford, England). 2020 Jun 1:59(6):1340-1346. doi: 10.1093/rheumatology/kez457. Epub
[PubMed PMID: 31593590]
[11]
Thorleifsdottir RH, Sigurdardottir SL, Sigurgeirsson B, Olafsson JH, Sigurdsson MI, Petersen H, Arnadottir S, Gudjonsson JE, Johnston A, Valdimarsson H. Improvement of psoriasis after tonsillectomy is associated with a decrease in the frequency of circulating T cells that recognize streptococcal determinants and homologous skin determinants. Journal of immunology (Baltimore, Md. : 1950). 2012 May 15:188(10):5160-5. doi: 10.4049/jimmunol.1102834. Epub 2012 Apr 9
[PubMed PMID: 22491250]
[12]
Thrastardottir T, Love TJ. Infections and the risk of psoriatic arthritis among psoriasis patients: a systematic review. Rheumatology international. 2018 Aug:38(8):1385-1397. doi: 10.1007/s00296-017-3873-4. Epub 2017 Nov 9
[PubMed PMID: 29124396]
Level 1 (high-level) evidence
[13]
Pattison E, Harrison BJ, Griffiths CE, Silman AJ, Bruce IN. Environmental risk factors for the development of psoriatic arthritis: results from a case-control study. Annals of the rheumatic diseases. 2008 May:67(5):672-6
[PubMed PMID: 17823200]
Level 2 (mid-level) evidence
[14]
Eder L, Law T, Chandran V, Shanmugarajah S, Shen H, Rosen CF, Cook RJ, Gladman DD. Association between environmental factors and onset of psoriatic arthritis in patients with psoriasis. Arthritis care & research. 2011 Aug:63(8):1091-7. doi: 10.1002/acr.20496. Epub
[PubMed PMID: 21560259]
[15]
Thorarensen SM, Lu N, Ogdie A, Gelfand JM, Choi HK, Love TJ. Physical trauma recorded in primary care is associated with the onset of psoriatic arthritis among patients with psoriasis. Annals of the rheumatic diseases. 2017 Mar:76(3):521-525. doi: 10.1136/annrheumdis-2016-209334. Epub 2016 Jul 25
[PubMed PMID: 27457510]
[16]
Poddubnyy D, Jadon DR, Van den Bosch F, Mease PJ, Gladman DD. Axial involvement in psoriatic arthritis: An update for rheumatologists. Seminars in arthritis and rheumatism. 2021 Aug:51(4):880-887. doi: 10.1016/j.semarthrit.2021.06.006. Epub 2021 Jun 19
[PubMed PMID: 34198146]
[17]
Ogdie A, Weiss P. The Epidemiology of Psoriatic Arthritis. Rheumatic diseases clinics of North America. 2015 Nov:41(4):545-68. doi: 10.1016/j.rdc.2015.07.001. Epub 2015 Sep 11
[PubMed PMID: 26476218]
[18]
Villani AP, Rouzaud M, Sevrain M, Barnetche T, Paul C, Richard MA, Beylot-Barry M, Misery L, Joly P, Le Maitre M, Aractingi S, Aubin F, Cantagrel A, Ortonne JP, Jullien D. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: Systematic review and meta-analysis. Journal of the American Academy of Dermatology. 2015 Aug:73(2):242-8. doi: 10.1016/j.jaad.2015.05.001. Epub 2015 Jun 6
[PubMed PMID: 26054432]
Level 1 (high-level) evidence
[19]
Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Annals of the rheumatic diseases. 2005 Mar:64 Suppl 2(Suppl 2):ii14-7
[PubMed PMID: 15708927]
[20]
Gladman DD, Shuckett R, Russell ML, Thorne JC, Schachter RK. Psoriatic arthritis (PSA)--an analysis of 220 patients. The Quarterly journal of medicine. 1987 Feb:62(238):127-41
[PubMed PMID: 3659255]
[21]
Eder L, Chandran V, Shen H, Cook RJ, Shanmugarajah S, Rosen CF, Gladman DD. Incidence of arthritis in a prospective cohort of psoriasis patients. Arthritis care & research. 2011 Apr:63(4):619-22. doi: 10.1002/acr.20401. Epub
[PubMed PMID: 21452273]
[22]
Wilson FC, Icen M, Crowson CS, McEvoy MT, Gabriel SE, Kremers HM. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population-based study. Arthritis and rheumatism. 2009 Feb 15:61(2):233-9. doi: 10.1002/art.24172. Epub
[PubMed PMID: 19177544]
[23]
Christophers E, Barker JN, Griffiths CE, Daudén E, Milligan G, Molta C, Sato R, Boggs R. The risk of psoriatic arthritis remains constant following initial diagnosis of psoriasis among patients seen in European dermatology clinics. Journal of the European Academy of Dermatology and Venereology : JEADV. 2010 May:24(5):548-54. doi: 10.1111/j.1468-3083.2009.03463.x. Epub 2009 Oct 23
[PubMed PMID: 19874432]
[24]
Stolwijk C, van Onna M, Boonen A, van Tubergen A. Global Prevalence of Spondyloarthritis: A Systematic Review and Meta-Regression Analysis. Arthritis care & research. 2016 Sep:68(9):1320-31. doi: 10.1002/acr.22831. Epub 2016 Jul 27
[PubMed PMID: 26713432]
Level 1 (high-level) evidence
[25]
Alinaghi F, Calov M, Kristensen LE, Gladman DD, Coates LC, Jullien D, Gottlieb AB, Gisondi P, Wu JJ, Thyssen JP, Egeberg A. Prevalence of psoriatic arthritis in patients with psoriasis: A systematic review and meta-analysis of observational and clinical studies. Journal of the American Academy of Dermatology. 2019 Jan:80(1):251-265.e19. doi: 10.1016/j.jaad.2018.06.027. Epub 2018 Jun 19
[PubMed PMID: 29928910]
Level 1 (high-level) evidence
[26]
Schett G, Lories RJ, D'Agostino MA, Elewaut D, Kirkham B, Soriano ER, McGonagle D. Enthesitis: from pathophysiology to treatment. Nature reviews. Rheumatology. 2017 Nov 21:13(12):731-741. doi: 10.1038/nrrheum.2017.188. Epub
[PubMed PMID: 29158573]
[27]
Ritchlin CT, Colbert RA, Gladman DD. Psoriatic Arthritis. The New England journal of medicine. 2017 May 25:376(21):2095-6. doi: 10.1056/NEJMc1704342. Epub
[PubMed PMID: 28538114]
[28]
McGonagle D, Gibbon W, Emery P. Classification of inflammatory arthritis by enthesitis. Lancet (London, England). 1998 Oct 3:352(9134):1137-40
[PubMed PMID: 9798608]
[29]
Sherlock JP, Joyce-Shaikh B, Turner SP, Chao CC, Sathe M, Grein J, Gorman DM, Bowman EP, McClanahan TK, Yearley JH, Eberl G, Buckley CD, Kastelein RA, Pierce RH, Laface DM, Cua DJ. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4-CD8- entheseal resident T cells. Nature medicine. 2012 Jul 1:18(7):1069-76. doi: 10.1038/nm.2817. Epub 2012 Jul 1
[PubMed PMID: 22772566]
[30]
Lories RJ, McInnes IB. Primed for inflammation: enthesis-resident T cells. Nature medicine. 2012 Jul 6:18(7):1018-9. doi: 10.1038/nm.2854. Epub 2012 Jul 6
[PubMed PMID: 22772553]
[31]
Veale DJ, Fearon U. The pathogenesis of psoriatic arthritis. Lancet (London, England). 2018 Jun 2:391(10136):2273-2284. doi: 10.1016/S0140-6736(18)30830-4. Epub 2018 Jun 1
[PubMed PMID: 29893226]
[32]
Stober C. Pathogenesis of psoriatic arthritis. Best practice & research. Clinical rheumatology. 2021 Jun:35(2):101694. doi: 10.1016/j.berh.2021.101694. Epub 2021 Jun 6
[PubMed PMID: 34108102]
[33]
Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H, CASPAR Study Group. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis and rheumatism. 2006 Aug:54(8):2665-73
[PubMed PMID: 16871531]
[34]
Jadon DR, Sengupta R, Nightingale A, Lindsay M, Korendowych E, Robinson G, Jobling A, Shaddick G, Bi J, Winchester R, Giles JT, McHugh NJ. Axial Disease in Psoriatic Arthritis study: defining the clinical and radiographic phenotype of psoriatic spondyloarthritis. Annals of the rheumatic diseases. 2017 Apr:76(4):701-707. doi: 10.1136/annrheumdis-2016-209853. Epub 2016 Dec 2
[PubMed PMID: 27913376]
[35]
Haddad A, Chandran V. Arthritis mutilans. Current rheumatology reports. 2013 Apr:15(4):321. doi: 10.1007/s11926-013-0321-7. Epub
[PubMed PMID: 23430715]
[36]
Chandran V, Gladman DD, Helliwell PS, Gudbjörnsson B. Arthritis mutilans: a report from the GRAPPA 2012 annual meeting. The Journal of rheumatology. 2013 Aug:40(8):1419-22. doi: 10.3899/jrheum.130453. Epub
[PubMed PMID: 23908536]
[37]
Belman S, Walsh JA, Carroll C, Milliken M, Haaland B, Duffin KC, Krueger GG, Feng BJ. Psoriasis Characteristics for the Early Detection of Psoriatic Arthritis. The Journal of rheumatology. 2021 Oct:48(10):1559-1565. doi: 10.3899/jrheum.201123. Epub 2021 Apr 15
[PubMed PMID: 33858978]
[38]
Cohen MR, Reda DJ, Clegg DO. Baseline relationships between psoriasis and psoriatic arthritis: analysis of 221 patients with active psoriatic arthritis. Department of Veterans Affairs Cooperative Study Group on Seronegative Spondyloarthropathies. The Journal of rheumatology. 1999 Aug:26(8):1752-6
[PubMed PMID: 10451073]
[39]
Williamson L, Dalbeth N, Dockerty JL, Gee BC, Weatherall R, Wordsworth BP. Extended report: nail disease in psoriatic arthritis--clinically important, potentially treatable and often overlooked. Rheumatology (Oxford, England). 2004 Jun:43(6):790-4
[PubMed PMID: 15113998]
[40]
Punzi L, Podswiadek M, Oliviero F, Lonigro A, Modesti V, Ramonda R, Todesco S. Laboratory findings in psoriatic arthritis. Reumatismo. 2007:59 Suppl 1():52-5
[PubMed PMID: 17828345]
[41]
Bogliolo L, Alpini C, Caporali R, Scirè CA, Moratti R, Montecucco C. Antibodies to cyclic citrullinated peptides in psoriatic arthritis. The Journal of rheumatology. 2005 Mar:32(3):511-5
[PubMed PMID: 15742445]
[42]
Silvy F, Bertin D, Bardin N, Auger I, Guzian MC, Mattei JP, Guis S, Roudier J, Balandraud N. Antinuclear Antibodies in Patients with Psoriatic Arthritis Treated or Not with Biologics. PloS one. 2015:10(7):e0134218. doi: 10.1371/journal.pone.0134218. Epub 2015 Jul 31
[PubMed PMID: 26230924]
[43]
Johnson SR, Schentag CT, Gladman DD. Autoantibodies in biological agent naive patients with psoriatic arthritis. Annals of the rheumatic diseases. 2005 May:64(5):770-2
[PubMed PMID: 15834057]
[45]
Siannis F, Farewell VT, Cook RJ, Schentag CT, Gladman DD. Clinical and radiological damage in psoriatic arthritis. Annals of the rheumatic diseases. 2006 Apr:65(4):478-81
[PubMed PMID: 16126794]
[46]
Kane D, Stafford L, Bresnihan B, FitzGerald O. A prospective, clinical and radiological study of early psoriatic arthritis: an early synovitis clinic experience. Rheumatology (Oxford, England). 2003 Dec:42(12):1460-8
[PubMed PMID: 14523223]
[47]
Sudoł-Szopińska I, Matuszewska G, Kwiatkowska B, Pracoń G. Diagnostic imaging of psoriatic arthritis. Part I: etiopathogenesis, classifications and radiographic features. Journal of ultrasonography. 2016 Mar:16(64):65-77. doi: 10.15557/JoU.2016.0007. Epub 2016 Mar 29
[PubMed PMID: 27104004]
[48]
Tan AL, Grainger AJ, Tanner SF, Emery P, McGonagle D. A high-resolution magnetic resonance imaging study of distal interphalangeal joint arthropathy in psoriatic arthritis and osteoarthritis: are they the same? Arthritis and rheumatism. 2006 Apr:54(4):1328-33
[PubMed PMID: 16575858]
[49]
Poggenborg RP, Østergaard M, Terslev L. Imaging in Psoriatic Arthritis. Rheumatic diseases clinics of North America. 2015 Nov:41(4):593-613. doi: 10.1016/j.rdc.2015.07.007. Epub 2015 Aug 25
[PubMed PMID: 26476221]
[50]
Offidani A, Cellini A, Valeri G, Giovagnoni A. Subclinical joint involvement in psoriasis: magnetic resonance imaging and X-ray findings. Acta dermato-venereologica. 1998 Nov:78(6):463-5
[PubMed PMID: 9833050]
[51]
Queiro R, Tejón P, Alonso S, Alperi M, Ballina J. Erosive discovertebral lesion (Andersson lesion) as the first sign of disease in axial psoriatic arthritis. Scandinavian journal of rheumatology. 2013:42(3):220-5. doi: 10.3109/03009742.2012.739637. Epub 2013 Jan 14
[PubMed PMID: 23311864]
[52]
Helliwell PS, Taylor WJ. Classification and diagnostic criteria for psoriatic arthritis. Annals of the rheumatic diseases. 2005 Mar:64 Suppl 2(Suppl 2):ii3-8
[PubMed PMID: 15708931]
[53]
Di Minno MN, Peluso R, Iervolino S, Russolillo A, Lupoli R, Scarpa R, CaRRDs Study Group. Weight loss and achievement of minimal disease activity in patients with psoriatic arthritis starting treatment with tumour necrosis factor α blockers. Annals of the rheumatic diseases. 2014 Jun:73(6):1157-62. doi: 10.1136/annrheumdis-2012-202812. Epub 2013 Jun 14
[PubMed PMID: 23771989]
[54]
Coates LC. Treating to target in psoriatic arthritis. Current opinion in rheumatology. 2015 Mar:27(2):107-10. doi: 10.1097/BOR.0000000000000140. Epub
[PubMed PMID: 25603035]
Level 3 (low-level) evidence
[55]
Coates LC, Moverley AR, McParland L, Brown S, Navarro-Coy N, O'Dwyer JL, Meads DM, Emery P, Conaghan PG, Helliwell PS. Effect of tight control of inflammation in early psoriatic arthritis (TICOPA): a UK multicentre, open-label, randomised controlled trial. Lancet (London, England). 2015 Dec 19:386(10012):2489-98. doi: 10.1016/S0140-6736(15)00347-5. Epub 2015 Oct 1
[PubMed PMID: 26433318]
Level 1 (high-level) evidence
[56]
Cuéllar ML, Citera G, Espinoza LR. Treatment of psoriatic arthritis. Bailliere's clinical rheumatology. 1994 May:8(2):483-98
[PubMed PMID: 8076399]
[57]
Lie E, van der Heijde D, Uhlig T, Heiberg MS, Koldingsnes W, Rødevand E, Kaufmann C, Mikkelsen K, Kvien TK. Effectiveness and retention rates of methotrexate in psoriatic arthritis in comparison with methotrexate-treated patients with rheumatoid arthritis. Annals of the rheumatic diseases. 2010 Apr:69(4):671-6. doi: 10.1136/ard.2009.113308. Epub 2009 Sep 9
[PubMed PMID: 19740904]
[58]
Singh JA, Guyatt G, Ogdie A, Gladman DD, Deal C, Deodhar A, Dubreuil M, Dunham J, Husni ME, Kenny S, Kwan-Morley J, Lin J, Marchetta P, Mease PJ, Merola JF, Miner J, Ritchlin CT, Siaton B, Smith BJ, Van Voorhees AS, Jonsson AH, Shah AA, Sullivan N, Turgunbaev M, Coates LC, Gottlieb A, Magrey M, Nowell WB, Orbai AM, Reddy SM, Scher JU, Siegel E, Siegel M, Walsh JA, Turner AS, Reston J. Special Article: 2018 American College of Rheumatology/National Psoriasis Foundation Guideline for the Treatment of Psoriatic Arthritis. Arthritis care & research. 2019 Jan:71(1):2-29. doi: 10.1002/acr.23789. Epub 2018 Nov 30
[PubMed PMID: 30499259]
[59]
Gossec L, Baraliakos X, Kerschbaumer A, de Wit M, McInnes I, Dougados M, Primdahl J, McGonagle DG, Aletaha D, Balanescu A, Balint PV, Bertheussen H, Boehncke WH, Burmester GR, Canete JD, Damjanov NS, Kragstrup TW, Kvien TK, Landewé RBM, Lories RJU, Marzo-Ortega H, Poddubnyy D, Rodrigues Manica SA, Schett G, Veale DJ, Van den Bosch FE, van der Heijde D, Smolen JS. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Annals of the rheumatic diseases. 2020 Jun:79(6):700-712. doi: 10.1136/annrheumdis-2020-217159. Epub
[PubMed PMID: 32434812]
[60]
Coates LC, Tillett W, Shaddick G, Pincus T, Kavanaugh A, Helliwell PS. Value of the Routine Assessment of Patient Index Data 3 in Patients With Psoriatic Arthritis: Results From a Tight-Control Clinical Trial and an Observational Cohort. Arthritis care & research. 2018 Aug:70(8):1198-1205. doi: 10.1002/acr.23460. Epub 2018 Jun 28
[PubMed PMID: 29112801]
[61]
Coates LC, Fransen J, Helliwell PS. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Annals of the rheumatic diseases. 2010 Jan:69(1):48-53. doi: 10.1136/ard.2008.102053. Epub
[PubMed PMID: 19147615]
[62]
FitzGerald O, Ogdie A, Chandran V, Coates LC, Kavanaugh A, Tillett W, Leung YY, deWit M, Scher JU, Mease PJ. Psoriatic arthritis. Nature reviews. Disease primers. 2021 Aug 12:7(1):59. doi: 10.1038/s41572-021-00293-y. Epub 2021 Aug 12
[PubMed PMID: 34385474]
[63]
Baraliakos X, Gossec L, Pournara E, Jeka S, Mera-Varela A, D'Angelo S, Schulz B, Rissler M, Nagar K, Perella C, Coates LC. Secukinumab in patients with psoriatic arthritis and axial manifestations: results from the double-blind, randomised, phase 3 MAXIMISE trial. Annals of the rheumatic diseases. 2021 May:80(5):582-590. doi: 10.1136/annrheumdis-2020-218808. Epub 2020 Dec 17
[PubMed PMID: 33334727]
Level 1 (high-level) evidence
[64]
Thomas ML, Shaddick G, Charlton R, Cavill C, Holland R, Iannone F, Lapadula G, Lopriore S, Závada J, Uher M, Pavelka K, Szczuková L, Sidiropoulos P, Flouri I, Drosos A, Möller B, Nissen MJ, Müller RB, Scherer A, McHugh N, Nightingale A. Tumor Necrosis Factor Inhibitor Monotherapy Versus Combination Therapy for the Treatment of Psoriatic Arthritis: Combined Analysis of European Biologics Databases. The Journal of rheumatology. 2021 Jan 1:48(1):48-57. doi: 10.3899/jrheum.190815. Epub 2020 Apr 1
[PubMed PMID: 32238520]
[65]
Lindström U, Di Giuseppe D, Delcoigne B, Glintborg B, Möller B, Ciurea A, Pombo-Suarez M, Sanchez-Piedra C, Eklund K, Relas H, Gudbjornsson B, Love TJ, Jones GT, Codreanu C, Ionescu R, Nekvindova L, Závada J, Atas N, Yolbas S, Fagerli KM, Michelsen B, Rotar Ž, Tomšič M, Iannone F, Santos MJ, Avila-Ribeiro P, Ørnbjerg LM, Østergaard M, Jacobsson LT, Askling J, Nissen MJ. Effectiveness and treatment retention of TNF inhibitors when used as monotherapy versus comedication with csDMARDs in 15 332 patients with psoriatic arthritis. Data from the EuroSpA collaboration. Annals of the rheumatic diseases. 2021 Nov:80(11):1410-1418. doi: 10.1136/annrheumdis-2021-220097. Epub 2021 Jun 3
[PubMed PMID: 34083206]
[66]
Acosta Felquer ML, LoGiudice L, Galimberti ML, Rosa J, Mazzuoccolo L, Soriano ER. Treating the skin with biologics in patients with psoriasis decreases the incidence of psoriatic arthritis. Annals of the rheumatic diseases. 2022 Jan:81(1):74-79. doi: 10.1136/annrheumdis-2021-220865. Epub 2021 Jul 19
[PubMed PMID: 34281904]
[67]
Gisondi P, Bellinato F, Targher G, Idolazzi L, Girolomoni G. Biological disease-modifying antirheumatic drugs may mitigate the risk of psoriatic arthritis in patients with chronic plaque psoriasis. Annals of the rheumatic diseases. 2022 Jan:81(1):68-73. doi: 10.1136/annrheumdis-2021-219961. Epub 2021 Jun 18
[PubMed PMID: 34144965]
[68]
Shalev Rosenthal Y, Schwartz N, Sagy I, Pavlovsky L. Reply. Arthritis & rheumatology (Hoboken, N.J.). 2022 Aug:74(8):1451-1452. doi: 10.1002/art.42123. Epub 2022 Jun 6
[PubMed PMID: 35315255]
[69]
Ytterberg SR, Bhatt DL, Connell CA. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. Reply. The New England journal of medicine. 2022 May 5:386(18):1768. doi: 10.1056/NEJMc2202778. Epub
[PubMed PMID: 35507493]
[70]
Ritchlin CT, Kavanaugh A, Gladman DD, Mease PJ, Helliwell P, Boehncke WH, de Vlam K, Fiorentino D, Fitzgerald O, Gottlieb AB, McHugh NJ, Nash P, Qureshi AA, Soriano ER, Taylor WJ, Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA). Treatment recommendations for psoriatic arthritis. Annals of the rheumatic diseases. 2009 Sep:68(9):1387-94. doi: 10.1136/ard.2008.094946. Epub 2008 Oct 24
[PubMed PMID: 18952643]
[71]
Gladman DD, Hing EN, Schentag CT, Cook RJ. Remission in psoriatic arthritis. The Journal of rheumatology. 2001 May:28(5):1045-8
[PubMed PMID: 11361187]
[72]
Gupta S, Syrimi Z, Hughes DM, Zhao SS. Comorbidities in psoriatic arthritis: a systematic review and meta-analysis. Rheumatology international. 2021 Feb:41(2):275-284. doi: 10.1007/s00296-020-04775-2. Epub 2021 Jan 9
[PubMed PMID: 33423070]
Level 1 (high-level) evidence
[73]
Gladman DD, Ang M, Su L, Tom BD, Schentag CT, Farewell VT. Cardiovascular morbidity in psoriatic arthritis. Annals of the rheumatic diseases. 2009 Jul:68(7):1131-5. doi: 10.1136/ard.2008.094839. Epub 2008 Aug 12
[PubMed PMID: 18697777]