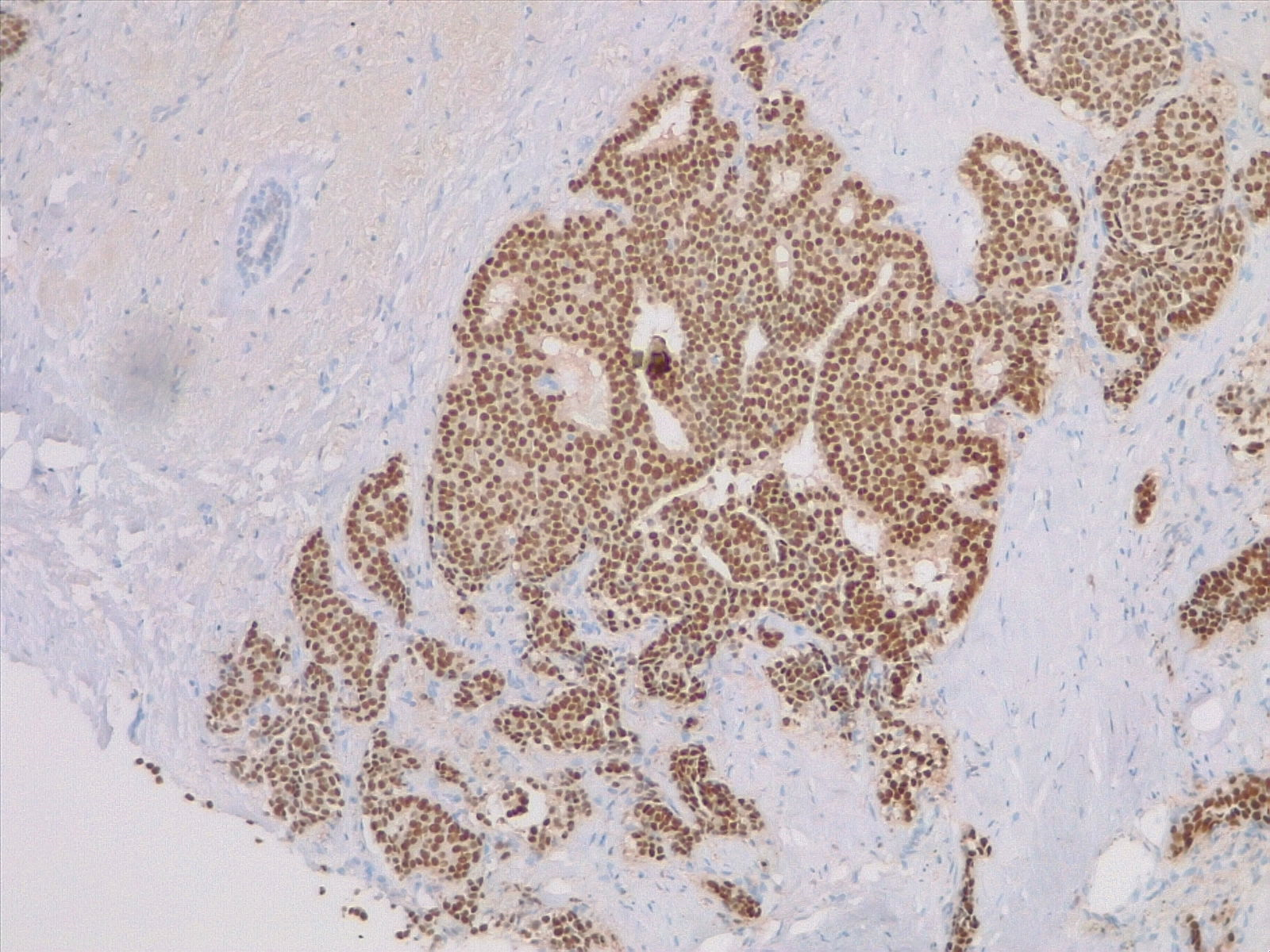
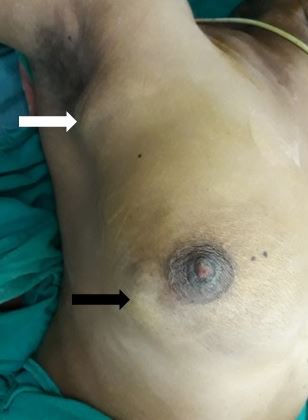
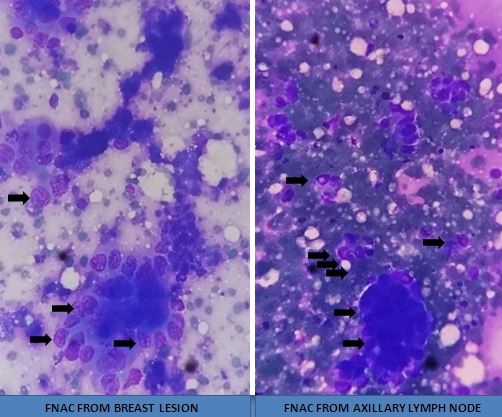
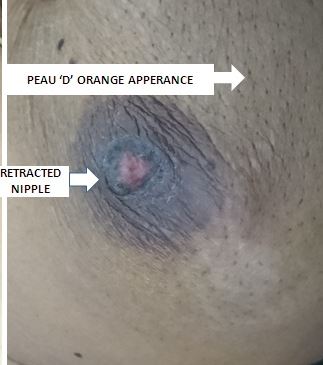
[1]
Mahvi DA, Liu R, Grinstaff MW, Colson YL, Raut CP. Local Cancer Recurrence: The Realities, Challenges, and Opportunities for New Therapies. CA: a cancer journal for clinicians. 2018 Nov:68(6):488-505. doi: 10.3322/caac.21498. Epub 2018 Oct 17
[PubMed PMID: 30328620]
[2]
Narod SA, Personalised medicine and population health: breast and ovarian cancer. Human genetics. 2018 Oct
[PubMed PMID: 30328515]
[3]
Cain EH, Saha A, Harowicz MR, Marks JR, Marcom PK, Mazurowski MA. Multivariate machine learning models for prediction of pathologic response to neoadjuvant therapy in breast cancer using MRI features: a study using an independent validation set. Breast cancer research and treatment. 2019 Jan:173(2):455-463. doi: 10.1007/s10549-018-4990-9. Epub 2018 Oct 16
[PubMed PMID: 30328048]
Level 1 (high-level) evidence
[4]
PDQ Screening and Prevention Editorial Board. Breast Cancer Screening (PDQ®): Health Professional Version. PDQ Cancer Information Summaries. 2002:():
[PubMed PMID: 26389344]
[5]
Doren A, Vecchiola A, Aguirre B, Villaseca P. Gynecological-endocrinological aspects in women carriers of BRCA1/2 gene mutations. Climacteric : the journal of the International Menopause Society. 2018 Dec:21(6):529-535. doi: 10.1080/13697137.2018.1514006. Epub 2018 Oct 8
[PubMed PMID: 30295091]
Level 2 (mid-level) evidence
[6]
Parada H Jr, Sun X, Tse CK, Olshan AF, Troester MA. Lifestyle Patterns and Survival Following Breast Cancer in the Carolina Breast Cancer Study. Epidemiology (Cambridge, Mass.). 2019 Jan:30(1):83-92. doi: 10.1097/EDE.0000000000000933. Epub
[PubMed PMID: 30299404]
[7]
White AJ, Bradshaw PT, Hamra GB. Air pollution and Breast Cancer: A Review. Current epidemiology reports. 2018 Jun:5(2):92-100. doi: 10.1007/s40471-018-0143-2. Epub 2018 Mar 27
[PubMed PMID: 30271702]
[8]
Gucalp A, Traina TA, Eisner JR, Parker JS, Selitsky SR, Park BH, Elias AD, Baskin-Bey ES, Cardoso F. Male breast cancer: a disease distinct from female breast cancer. Breast cancer research and treatment. 2019 Jan:173(1):37-48. doi: 10.1007/s10549-018-4921-9. Epub 2018 Sep 28
[PubMed PMID: 30267249]
[9]
Clark BZ, Onisko A, Assylbekova B, Li X, Bhargava R, Dabbs DJ. Breast cancer global tumor biomarkers: a quality assurance study of intratumoral heterogeneity. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2019 Mar:32(3):354-366. doi: 10.1038/s41379-018-0153-0. Epub 2018 Oct 16
[PubMed PMID: 30327501]
Level 2 (mid-level) evidence
[10]
Liedtke C, Kolberg HC, Kerschke L, Görlich D, Bauerfeind I, Fehm T, Fleige B, Helms G, Lebeau A, Stäbler A, Schmatloch S, Hausschild M, Schwentner L, von Minckwitz G, Loibl S, Untch M, Kühn T. Systematic analysis of parameters predicting pathological axillary status (ypN0 vs. ypN+) in patients with breast cancer converting from cN+ to ycN0 through primary systemic therapy (PST). Clinical & experimental metastasis. 2018 Dec:35(8):777-783. doi: 10.1007/s10585-018-9938-2. Epub 2018 Oct 15
[PubMed PMID: 30324492]
Level 1 (high-level) evidence
[11]
Kitamura M, Nakayama T, Mukaisho KI, Mori T, Umeda T, Moritani S, Kushima R, Tani M, Sugihara H. Progression Potential of Ductal Carcinoma in situ Assessed by Genomic Copy Number Profiling. Pathobiology : journal of immunopathology, molecular and cellular biology. 2019:86(2-3):92-101. doi: 10.1159/000492833. Epub 2018 Oct 17
[PubMed PMID: 30332671]
[12]
Radovic N, Ivanac G, Divjak E, Biondic I, Bulum A, Brkljacic B. Evaluation of Breast Cancer Morphology Using Diffusion-Weighted and Dynamic Contrast-Enhanced MRI: Intermethod and Interobserver Agreement. Journal of magnetic resonance imaging : JMRI. 2019 May:49(5):1381-1390. doi: 10.1002/jmri.26332. Epub 2018 Oct 16
[PubMed PMID: 30325549]
[13]
Pediconi F, Marzocca F, Cavallo Marincola B, Napoli A. MRI-guided treatment in the breast. Journal of magnetic resonance imaging : JMRI. 2018 Dec:48(6):1479-1488. doi: 10.1002/jmri.26282. Epub 2018 Oct 14
[PubMed PMID: 30318672]
[14]
Watanabe Y, Anan K. The decision to perform or omit sentinel lymph node biopsy during mastectomy for ductal carcinoma in situ should be tailored in accordance with preoperative findings. Breast cancer (Tokyo, Japan). 2019 Mar:26(2):261-262. doi: 10.1007/s12282-018-0917-x. Epub 2018 Oct 16
[PubMed PMID: 30328007]
[15]
Rocque GB, Williams CP, Kenzik KM, Jackson BE, Azuero A, Halilova KI, Ingram SA, Pisu M, Forero A, Bhatia S. Concordance with NCCN treatment guidelines: Relations with health care utilization, cost, and mortality in breast cancer patients with secondary metastasis. Cancer. 2018 Nov 1:124(21):4231-4240. doi: 10.1002/cncr.31694. Epub 2018 Oct 14
[PubMed PMID: 30317547]
[16]
Vande Perre P, Toledano D, Corsini C, Escriba E, Laporte M, Bertet H, Yauy K, Toledano A, Galibert V, Baudry K, Clotet L, Million E, Picot MC, Geneviève D, Pujol P. Role of the general practitioner in the care of BRCA1 and BRCA2 mutation carriers: General practitioner and patient perspectives. Molecular genetics & genomic medicine. 2018 Nov:6(6):957-965. doi: 10.1002/mgg3.464. Epub 2018 Oct 11
[PubMed PMID: 30308700]
Level 3 (low-level) evidence
[17]
Seroussi B, Lamy JB, Muro N, Larburu N, Sekar BD, Guézennec G, Bouaud J. Implementing Guideline-Based, Experience-Based, and Case-Based Approaches to Enrich Decision Support for the Management of Breast Cancer Patients in the DESIREE Project. Studies in health technology and informatics. 2018:255():190-194
[PubMed PMID: 30306934]
Level 3 (low-level) evidence
[18]
Wang X, Xu L, Yin Z, Wang D, Wang Q, Xu K, Zhao J, Zhao L, Yuan Z, Wang P. Locoregional recurrence-associated factors and risk-adapted postmastectomy radiotherapy for breast cancer staged in cT1-2N0-1 after neoadjuvant chemotherapy. Cancer management and research. 2018:10():4105-4112. doi: 10.2147/CMAR.S173628. Epub 2018 Oct 2
[PubMed PMID: 30323666]
[19]
Tang L, Matsushita H, Jingu K. Controversial issues in radiotherapy after breast-conserving surgery for early breast cancer in older patients: a systematic review. Journal of radiation research. 2018 Nov 1:59(6):789-793. doi: 10.1093/jrr/rry071. Epub
[PubMed PMID: 30321392]
Level 1 (high-level) evidence
[20]
Wu YT, Xu Z, Zhang K, Wu JS, Li X, Arshad B, Li YC, Wang ZL, Li HY, Wu KN, Kong LQ. Efficacy and cardiac safety of the concurrent use of trastuzumab and anthracycline-based neoadjuvant chemotherapy for HER2-positive breast cancer: a systematic review and meta-analysis. Therapeutics and clinical risk management. 2018:14():1789-1797. doi: 10.2147/TCRM.S176214. Epub 2018 Sep 26
[PubMed PMID: 30310287]
Level 1 (high-level) evidence
[21]
Dieci MV, Vernaci G, Guarneri V. Escalation and de-escalation in HER2 positive early breast cancer. Current opinion in oncology. 2019 Jan:31(1):35-42. doi: 10.1097/CCO.0000000000000492. Epub
[PubMed PMID: 30325338]
Level 3 (low-level) evidence
[22]
Hodis HN, Sarrel PM. Menopausal hormone therapy and breast cancer: what is the evidence from randomized trials? Climacteric : the journal of the International Menopause Society. 2018 Dec:21(6):521-528. doi: 10.1080/13697137.2018.1514008. Epub 2018 Oct 9
[PubMed PMID: 30296850]
Level 1 (high-level) evidence
[23]
Gautam S, Sylwestrzak G, Barron J, Chen X, Eleff M, Debono D, Nguyen A, Fisch M. Results From a Health Insurer's Clinical Pathway Program in Breast Cancer. Journal of oncology practice. 2018 Oct 15:():JOP1800157. doi: 10.1200/JOP.18.00157. Epub 2018 Oct 15
[PubMed PMID: 30321101]
[24]
Wapnir IL, Dignam JJ, Fisher B, Mamounas EP, Anderson SJ, Julian TB, Land SR, Margolese RG, Swain SM, Costantino JP, Wolmark N. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. Journal of the National Cancer Institute. 2011 Mar 16:103(6):478-88. doi: 10.1093/jnci/djr027. Epub 2011 Mar 11
[PubMed PMID: 21398619]
Level 1 (high-level) evidence
[25]
Litière S, Werutsky G, Fentiman IS, Rutgers E, Christiaens MR, Van Limbergen E, Baaijens MH, Bogaerts J, Bartelink H. Breast conserving therapy versus mastectomy for stage I-II breast cancer: 20 year follow-up of the EORTC 10801 phase 3 randomised trial. The Lancet. Oncology. 2012 Apr:13(4):412-9. doi: 10.1016/S1470-2045(12)70042-6. Epub 2012 Feb 27
[PubMed PMID: 22373563]
Level 1 (high-level) evidence
[26]
Kirby AM. Updated ASTRO guidelines on accelerated partial breast irradiation (APBI): to whom can we offer APBI outside a clinical trial? The British journal of radiology. 2018 May:91(1085):20170565. doi: 10.1259/bjr.20170565. Epub 2018 Mar 23
[PubMed PMID: 29513031]
[27]
Vicini FA, Cecchini RS, White JR, Arthur DW, Julian TB, Rabinovitch RA, Kuske RR, Ganz PA, Parda DS, Scheier MF, Winter KA, Paik S, Kuerer HM, Vallow LA, Pierce LJ, Mamounas EP, McCormick B, Costantino JP, Bear HD, Germain I, Gustafson G, Grossheim L, Petersen IA, Hudes RS, Curran WJ Jr, Bryant JL, Wolmark N. Long-term primary results of accelerated partial breast irradiation after breast-conserving surgery for early-stage breast cancer: a randomised, phase 3, equivalence trial. Lancet (London, England). 2019 Dec 14:394(10215):2155-2164. doi: 10.1016/S0140-6736(19)32514-0. Epub 2019 Dec 5
[PubMed PMID: 31813636]
Level 1 (high-level) evidence
[28]
Bartelink H, Horiot JC, Poortmans PM, Struikmans H, Van den Bogaert W, Fourquet A, Jager JJ, Hoogenraad WJ, Oei SB, Wárlám-Rodenhuis CC, Pierart M, Collette L. Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10-year results of the randomized boost versus no boost EORTC 22881-10882 trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007 Aug 1:25(22):3259-65
[PubMed PMID: 17577015]
Level 1 (high-level) evidence
[29]
Overgaard M, Nielsen HM, Tramm T, Højris I, Grantzau TL, Alsner J, Offersen BV, Overgaard J, DBCG Radiotherapy Group. Postmastectomy radiotherapy in high-risk breast cancer patients given adjuvant systemic therapy. A 30-year long-term report from the Danish breast cancer cooperative group DBCG 82bc trial. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2022 May:170():4-13. doi: 10.1016/j.radonc.2022.03.008. Epub 2022 Mar 11
[PubMed PMID: 35288227]
[30]
Donker M, van Tienhoven G, Straver ME, Meijnen P, van de Velde CJ, Mansel RE, Cataliotti L, Westenberg AH, Klinkenbijl JH, Orzalesi L, Bouma WH, van der Mijle HC, Nieuwenhuijzen GA, Veltkamp SC, Slaets L, Duez NJ, de Graaf PW, van Dalen T, Marinelli A, Rijna H, Snoj M, Bundred NJ, Merkus JW, Belkacemi Y, Petignat P, Schinagl DA, Coens C, Messina CG, Bogaerts J, Rutgers EJ. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. The Lancet. Oncology. 2014 Nov:15(12):1303-10. doi: 10.1016/S1470-2045(14)70460-7. Epub 2014 Oct 15
[PubMed PMID: 25439688]
Level 1 (high-level) evidence
[31]
Whelan TJ, Olivotto IA, Parulekar WR, Ackerman I, Chua BH, Nabid A, Vallis KA, White JR, Rousseau P, Fortin A, Pierce LJ, Manchul L, Chafe S, Nolan MC, Craighead P, Bowen J, McCready DR, Pritchard KI, Gelmon K, Murray Y, Chapman JA, Chen BE, Levine MN, MA.20 Study Investigators. Regional Nodal Irradiation in Early-Stage Breast Cancer. The New England journal of medicine. 2015 Jul 23:373(4):307-16. doi: 10.1056/NEJMoa1415340. Epub
[PubMed PMID: 26200977]
[32]
Choi KH, Ahn SJ, Jeong JU, Yu M, Kim JH, Jeong BK, Lee JH, Kim SH, Lee JH. Postoperative radiotherapy with intensity-modulated radiation therapy versus 3-dimensional conformal radiotherapy in early breast cancer: A randomized clinical trial of KROG 15-03. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2021 Jan:154():179-186. doi: 10.1016/j.radonc.2020.09.043. Epub 2020 Sep 24
[PubMed PMID: 32980384]
Level 1 (high-level) evidence
[33]
Pignol JP, Truong P, Rakovitch E, Sattler MG, Whelan TJ, Olivotto IA. Ten years results of the Canadian breast intensity modulated radiation therapy (IMRT) randomized controlled trial. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2016 Dec:121(3):414-419. doi: 10.1016/j.radonc.2016.08.021. Epub 2016 Sep 13
[PubMed PMID: 27637858]
Level 1 (high-level) evidence
[34]
Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, Correa C, Cutter D, Gagliardi G, Gigante B, Jensen MB, Nisbet A, Peto R, Rahimi K, Taylor C, Hall P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. The New England journal of medicine. 2013 Mar 14:368(11):987-98. doi: 10.1056/NEJMoa1209825. Epub
[PubMed PMID: 23484825]
[35]
Epler GR, Kelly EM. Systematic review of postradiotherapy bronchiolitis obliterans organizing pneumonia in women with breast cancer. The oncologist. 2014 Dec:19(12):1216-26. doi: 10.1634/theoncologist.2014-0041. Epub 2014 Oct 31
[PubMed PMID: 25361622]
Level 1 (high-level) evidence
[36]
Taghian AG, Assaad SI, Niemierko A, Floyd SR, Powell SN. Is a reduction in radiation lung volume and dose necessary with paclitaxel chemotherapy for node-positive breast cancer? International journal of radiation oncology, biology, physics. 2005 Jun 1:62(2):386-91
[PubMed PMID: 15890579]
[37]
Williams NR, Williams S, Kanapathy M, Naderi N, Vavourakis V, Mosahebi A. Radiation-induced fibrosis in breast cancer: A protocol for an observational cross-sectional pilot study for personalised risk estimation and objective assessment. International journal of surgery protocols. 2019:14():9-13. doi: 10.1016/j.isjp.2019.02.002. Epub 2019 Feb 13
[PubMed PMID: 31851743]
Level 2 (mid-level) evidence
[38]
Collette S, Collette L, Budiharto T, Horiot JC, Poortmans PM, Struikmans H, Van den Bogaert W, Fourquet A, Jager JJ, Hoogenraad W, Mueller RP, Kurtz J, Morgan DA, Dubois JB, Salamon E, Mirimanoff R, Bolla M, Van der Hulst M, Wárlám-Rodenhuis CC, Bartelink H, EORTC Radiation Oncology Group. Predictors of the risk of fibrosis at 10 years after breast conserving therapy for early breast cancer: a study based on the EORTC Trial 22881-10882 'boost versus no boost'. European journal of cancer (Oxford, England : 1990). 2008 Nov:44(17):2587-99. doi: 10.1016/j.ejca.2008.07.032. Epub 2008 Aug 29
[PubMed PMID: 18757193]
[39]
Jacobson G, Bhatia S, Smith BJ, Button AM, Bodeker K, Buatti J. Randomized trial of pentoxifylline and vitamin E vs standard follow-up after breast irradiation to prevent breast fibrosis, evaluated by tissue compliance meter. International journal of radiation oncology, biology, physics. 2013 Mar 1:85(3):604-8. doi: 10.1016/j.ijrobp.2012.06.042. Epub 2012 Jul 28
[PubMed PMID: 22846413]
Level 1 (high-level) evidence
[40]
Gross JP, Whelan TJ, Parulekar WR, Chen BE, Rademaker AW, Helenowski IB, Donnelly ED, Strauss JB. Development and Validation of a Nomogram to Predict Lymphedema After Axillary Surgery and Radiation Therapy in Women With Breast Cancer From the NCIC CTG MA.20 Randomized Trial. International journal of radiation oncology, biology, physics. 2019 Sep 1:105(1):165-173. doi: 10.1016/j.ijrobp.2019.05.002. Epub 2019 May 11
[PubMed PMID: 31085285]
Level 1 (high-level) evidence
[41]
DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. The Lancet. Oncology. 2013 May:14(6):500-15. doi: 10.1016/S1470-2045(13)70076-7. Epub 2013 Mar 27
[PubMed PMID: 23540561]
Level 1 (high-level) evidence
[42]
Bartels SAL, Donker M, Poncet C, Sauvé N, Straver ME, van de Velde CJH, Mansel RE, Blanken C, Orzalesi L, Klinkenbijl JHG, van der Mijle HCJ, Nieuwenhuijzen GAP, Veltkamp SC, van Dalen T, Marinelli A, Rijna H, Snoj M, Bundred NJ, Merkus JWS, Belkacemi Y, Petignat P, Schinagl DAX, Coens C, van Tienhoven G, van Duijnhoven F, Rutgers EJT. Radiotherapy or Surgery of the Axilla After a Positive Sentinel Node in Breast Cancer: 10-Year Results of the Randomized Controlled EORTC 10981-22023 AMAROS Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2023 Apr 20:41(12):2159-2165. doi: 10.1200/JCO.22.01565. Epub 2022 Nov 16
[PubMed PMID: 36383926]
Level 1 (high-level) evidence
[43]
Pierce SM, Recht A, Lingos TI, Abner A, Vicini F, Silver B, Herzog A, Harris JR. Long-term radiation complications following conservative surgery (CS) and radiation therapy (RT) in patients with early stage breast cancer. International journal of radiation oncology, biology, physics. 1992:23(5):915-23
[PubMed PMID: 1639653]
[44]
Meric F, Buchholz TA, Mirza NQ, Vlastos G, Ames FC, Ross MI, Pollock RE, Singletary SE, Feig BW, Kuerer HM, Newman LA, Perkins GH, Strom EA, McNeese MD, Hortobagyi GN, Hunt KK. Long-term complications associated with breast-conservation surgery and radiotherapy. Annals of surgical oncology. 2002 Jul:9(6):543-9
[PubMed PMID: 12095969]
[45]
Grantzau T, Overgaard J. Risk of second non-breast cancer after radiotherapy for breast cancer: a systematic review and meta-analysis of 762,468 patients. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2015 Jan:114(1):56-65. doi: 10.1016/j.radonc.2014.10.004. Epub 2014 Nov 7
[PubMed PMID: 25454172]
Level 1 (high-level) evidence
[46]
Ye JC, Yan W, Christos P, Nori D, Chao KS, Ravi A. Second cancer, breast cancer, and cardiac mortality in stage T1aN0 breast cancer patients with or without external beam radiation therapy: a national registry study. Clinical breast cancer. 2015 Feb:15(1):54-9. doi: 10.1016/j.clbc.2014.07.003. Epub 2014 Aug 15
[PubMed PMID: 25223278]
[47]
Dracham CB, Shankar A, Madan R. Radiation induced secondary malignancies: a review article. Radiation oncology journal. 2018 Jun:36(2):85-94. doi: 10.3857/roj.2018.00290. Epub 2018 Jun 29
[PubMed PMID: 29983028]