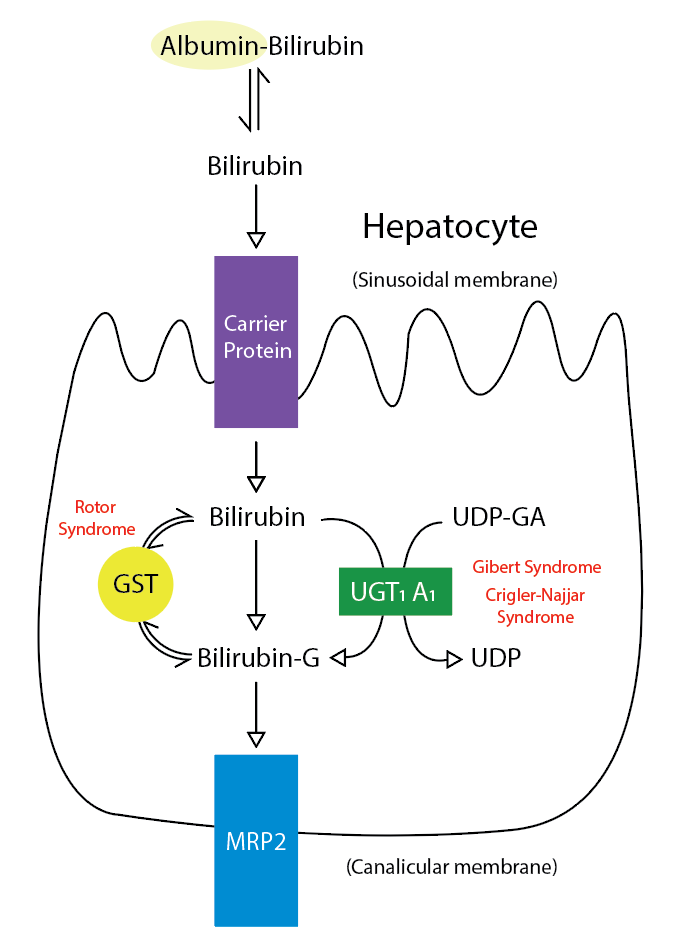
Introduction
Bilirubin is an important metabolite of heme (ferroprotoporphyrin IX), a coordination complex that serves to coordinate iron in various proteins. It is a potentially toxic substance. However, the body has developed mechanisms for its safe detoxification and disposition. Bilirubin and its metabolites also provide the distinctive yellow color to bile and stool and a lesser degree, urine. This article will summarize the mechanism of heme metabolism and bilirubin synthesis.[1][2][3]
Formation of Bilirubin
Bilirubin is derived from two main sources. Roughly, 80% of bilirubin is made from the breakdown of hemoglobin in senescent red blood cells, and prematurely destroyed erythroid cells in the bone marrow. The remainder originates from the turnover of various heme-containing proteins found in other tissues, primarily the liver and muscles. These proteins include myoglobin, cytochromes, catalase, peroxidase, and tryptophan pyrrolase.[4][5] About 4 mg/kg body weight of bilirubin is produced daily.